Euler's constant is also approximately equal to a half. But 60/122 is certainly easier to remember and almost makes sense. OTOH 1/2 is pretty easy to remember too though determining why the answer is 0.5 is more difficult than following Martin's explanation.60/122 = 0.4918, and that factor actually covers nigh on all generally encountered source water pH's rather well per A.J.'s chart. Seems even more practical for use than 0.5, but admittedly this is merely hair splitting.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Please advise on my tap water suitability for brewing.
- Thread starter MajorJC
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
couchsending
Well-Known Member
- Joined
- Jun 21, 2016
- Messages
- 3,063
- Reaction score
- 2,256
Wow talk about a tangent... poor OP just wanted a simple answer. Haha
RPh_Guy
Bringing Sour Back
"What should I add to my water?" is kind of like asking "what malt should I use to make beer?"Wow talk about a tangent... poor OP just wanted a simple answer. Haha
Yes, well, um.... It looks as if I was deceiving you (and myself) a bit there. It turns out after all that the factor does go up appreciably at higher pH. Here's how things look as corrected and presented in what I hope is a much clearer way:Thanks A.J.! The deviation from 0.5 is overall far less than your initial post #16 had indicated. I'm happy with using 0.50.
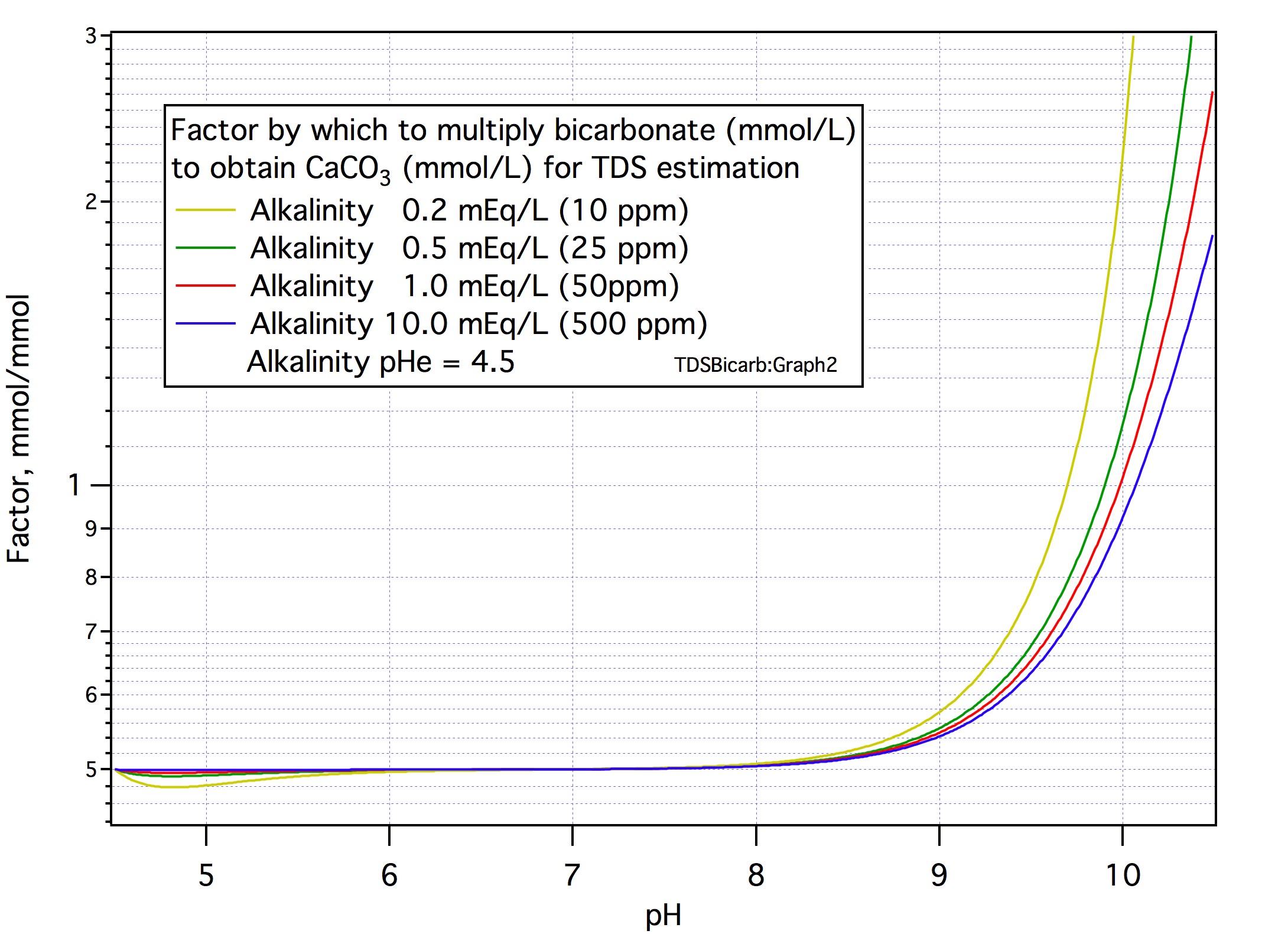
Let's be absolutely clear about what this graph shows. It gives values for a factor by which you multiply the mmol/L bicarbonate as given on a water report to get the mmol/L CaCO3 that was dissolved by nature (or whomever) to give you the water you have in hand. Put in other words if you multiply the reported bicarbonate in mg/L by this factor and then multiply the result by (100/61) the result is the mg/L CaCO3 that was originally dissolved and, therefore, the amount of CaCO3 you would find in the residue if you evaporated a liter of the water.
Therefore, to use this factor to estimate gravimetric TDS you would
1) Add up the masses of all the ions in the report except HCO3- and CO3--
2) Multiply the reported bicarbonate (mg/L) by (100/61) times the factor
3) Add that to the sum obtained in 1.
As the curves show the factor is 0.5 up to pH 8 and pretty close to it up to pH 9. Note that the deviation is largest when the alkalinity is smallest which is, of course, when there isn't much limestone dissolved in the water anyway and continuing to use 0.5 will cause only a small error because of that. Above pH 9 you'll have to make your own determination. Note that this is in the region where the bicarbonate = 61/50 approximation used by so many 1st Gen calculators is starting to fall apart so you don't have a good bicarbonate number to work with. A Gen II calculator never even bothers to calculate bicarbonate determining the amount of lime initially dissolved directly from the alkalinity and sample pH.
Now there is a BIG caveat that goes with this. Suppose you put 100 mg of limestone in a liter of water and added HCl to dissolve it and adjust the pH to 8.4. At that point you would have about 1 mmol of bicarbonate ions and an alkalinity of about 1 mEq/L. If you put pH = 8.4 and 1 mEq/L into the factor formula it is, as the curves show, going to give you a factor of a little over 0.5. Multiplying that by the 1 mmol/L bicarbonate would tell you that half a mmol of limestone (50 mg) were dissolved. That isn't right, of course. Thus the use of this factor is, for the moment, only applicable where the water is natural. Clearly in this simple case we can detect this situation by observing that the temporary hardness is about equal to the total hardness and there may be a work around for the more general case based on this. An area for some further thought at another time and place.
For now the function to compute the factor in natural water has been added to the Voltmeter spreadsheet (brewingfunctions.yolasite.com). I've also put a note on how to calculate it there.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Thanks again A.J.!!!
But now my head is really spinning.
But now my head is really spinning.
And speaking of tangents: does anyone still use metal pipe?However most wouldn't be softening to almost zero hardness like this sample shows. That is hell on your metal pipes.

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
Guangshui Weilu You Trading Co., Ltd

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid MFL)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$27.29 ($13.64 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$28.98
Five Star - 6022b_ - Star San - 32 Ounce - High Foaming Sanitizer
Great Fermentations of Indiana

$172.35
2 Inch Tri Clamp Keg Manifold With Ball Lock Posts, Pressure Gauge, PRV (0-30 PSI) – Homebrew, Fermentation, Kegging System
wuhanshijiayangzhiyimaoyiyouxiangongsi

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
And speaking of tangents: does anyone still use metal pipe?
I do! I guess it's time for me to get a pH reading on my softened water. I know my well water is ~7.2 pH.
Well I did go around cock robin's barn here quite a bit and was really coming at it from the wrong end in No 25. Go to yolasite and get the note. It might make things a little clearer. I didn't want to take up more space here as most readers wouldn't be in the least interested.But now my head is really spinning.
We'll also need the post softener alkalinity (I assume no neutralizer with pH 7.2) and the post softener hardness (which we can wag at 1 or 2 mg/L). Don't be too concerned at this point. Low post softener calcium isn't really hell on metal pipes. It isn't heaven but it probably isn't hell either. I bought an old house with a softener and metal pipes and never had a problem even after living in it for years. The only pinhole leak I ever experienced was with new plumbing installed after I took the softener out. It was simply defective pipe (in a chase, of course).I do! I guess it's time for me to get a pH reading on my softened water. I know my well water is ~7.2 pH.
OP... This is what i have done ever since I got my water report.....Use RO and follow guidance below. Instead of using sauermalz I drop in a tsp or 2 of lactic acid (it’s just easier) to get PH in range for lighter beers. I also use a water pgm like brew-n-water (brewers friend actually) but you may not want to mess with it...up to you.
Sorry your tap water is sketchy for brewing...good luck!
https://www.homebrewtalk.com/forum/threads/a-brewing-water-chemistry-primer.198460/
The following recommendations apply to “soft” water. Here we will define soft as meaning RO or distilled water or any water whose lab report indicates alkalinity less than 35 (ppm as CaCO3 – all other numbers to follow mg/L), sulfate less than 20 (as sulfate – Ward Labs reports as sulfur so multiply the SO4-S number by 3 to get as sulfate), chloride less than 20, sodium less than 20, calcium less than 20 and magnesium less than 20. If your water has numbers higher than these, dilute it with RO or DI water. A 1:1 dilution reduces each ion concentration to 1/2, a 2:1 dilution to 1/3 and so on. If your water contains chloramines add 1 campden tablet per 20 gallons (before any dilution)
Baseline: Add 1 tsp of calcium chloride dihydrate (what your LHBS sells) to each 5 gallons of water treated. Add 2% sauermalz to the grist.
Deviate from the baseline as follows:
For soft water beers (i.e Pils, Helles). Use half the baseline amount of calcium chloride and increase the sauermalz to 3%
For beers that use roast malt (Stout, porter): Skip the sauermalz.
For British beers: Add 1 tsp gypsum as well as 1 tsp calcium chloride
For very minerally beers (Export, Burton ale): Double the calcium chloride and the gypsum.
Sorry your tap water is sketchy for brewing...good luck!
https://www.homebrewtalk.com/forum/threads/a-brewing-water-chemistry-primer.198460/
The following recommendations apply to “soft” water. Here we will define soft as meaning RO or distilled water or any water whose lab report indicates alkalinity less than 35 (ppm as CaCO3 – all other numbers to follow mg/L), sulfate less than 20 (as sulfate – Ward Labs reports as sulfur so multiply the SO4-S number by 3 to get as sulfate), chloride less than 20, sodium less than 20, calcium less than 20 and magnesium less than 20. If your water has numbers higher than these, dilute it with RO or DI water. A 1:1 dilution reduces each ion concentration to 1/2, a 2:1 dilution to 1/3 and so on. If your water contains chloramines add 1 campden tablet per 20 gallons (before any dilution)
Baseline: Add 1 tsp of calcium chloride dihydrate (what your LHBS sells) to each 5 gallons of water treated. Add 2% sauermalz to the grist.
Deviate from the baseline as follows:
For soft water beers (i.e Pils, Helles). Use half the baseline amount of calcium chloride and increase the sauermalz to 3%
For beers that use roast malt (Stout, porter): Skip the sauermalz.
For British beers: Add 1 tsp gypsum as well as 1 tsp calcium chloride
For very minerally beers (Export, Burton ale): Double the calcium chloride and the gypsum.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
We'll also need the post softener alkalinity (I assume no neutralizer with pH 7.2) and the post softener hardness (which we can wag at 1 or 2 mg/L). Don't be too concerned at this point. Low post softener calcium isn't really hell on metal pipes. It isn't heaven but it probably isn't hell either. I bought an old house with a softener and metal pipes and never had a problem even after living in it for years. The only pinhole leak I ever experienced was with new plumbing installed after I took the softener out. It was simply defective pipe (in a chase, of course).
The well water and the softened well water both have essentially the same TDS (via meter) of around 870. From a GH/KH test kit I'm pretty sure my well waters alkalinity is ~437 ppm. I never did this test for the softened water, as I have always presumed it is still 437 ppm alkalinity.
The GH/KH test (rounded from multiple tries for the well water) was:
~42.5 drops (avg.) for GH
~24.5 drops (avg.) for KH
And I believe that ppm = 17.848 x dH
Therefore:
24.5 drops x 17.848 = 437 ppm alkalinity
42.5 drops x 17.848 = 758.5 ppm total hardness
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
If I presume my well water to have the following make-up, I get nigh on perfect cation/anion balance, and also 759 ppm total hardness, and lastly 860 summed ions method TDS for the following wild + educated guess at what may be looming within my well water:
Ca = 212.4 ppm
Mg = 55.4 ppm
Na = 11 ppm
Cl = 45 ppm
SO4 = 270 ppm
Alkalinity = 437 ppm
Bicarb = 533 ppm
pH = 7.2 (by meter)
The well water wreaks of sulfur odor, which dissipates to undetectable (by nose) if left to sit out in an open container to air itself out for a few days.
I only used this water once, at 25% well and 75% RO, and the Vienna Lager I made from it turned out fairly decent. Nothing to write home about, but it was about mainstream normal for my efforts. It took a fair amount of acidification to knock out the alkalinity and bring the water to ~pH 5.6, even at only 25% well. that was a few years ago. I won't mess with it again.
Ca = 212.4 ppm
Mg = 55.4 ppm
Na = 11 ppm
Cl = 45 ppm
SO4 = 270 ppm
Alkalinity = 437 ppm
Bicarb = 533 ppm
pH = 7.2 (by meter)
The well water wreaks of sulfur odor, which dissipates to undetectable (by nose) if left to sit out in an open container to air itself out for a few days.
I only used this water once, at 25% well and 75% RO, and the Vienna Lager I made from it turned out fairly decent. Nothing to write home about, but it was about mainstream normal for my efforts. It took a fair amount of acidification to knock out the alkalinity and bring the water to ~pH 5.6, even at only 25% well. that was a few years ago. I won't mess with it again.
Last edited:
Wow! But we need the post softener calcium hardness. Unless it's above 40 ppm your saturation pH is going to be higher than your water's pH and no protective film will deposit. But the water's pH is 7.2. The H+ ion content is less than 1E-4 mEq/L. It's going to take that water a long time to corrode your metal pipes. How long have you lived with this system? Have you ever seen any evidence of corrosion? Put a piece of copper wire in hydrochloric acid (I assume you have copper pipe). What happens? Check the copper content of your tap water. Is it significant?
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Wow! But we need the post softener calcium hardness. Unless it's above 40 ppm your saturation pH is going to be higher than your water's pH and no protective film will deposit. But the water's pH is 7.2. The H+ ion content is less than 1E-4 mEq/L. It's going to take that water a long time to corrode your metal pipes. How long have you lived with this system? Have you ever seen any evidence of corrosion? Put a piece of copper wire in hydrochloric acid (I assume you have copper pipe). What happens? Check the copper content of your tap water. Is it significant?
Should I see if I can find the GH/KH test kit, and have a go at my softened water with it?
After it passes through our under sink RO unit, the TDS is about 42 to 45 ppm via the same meter that gave me roughly 876 TDS for both the well and softened water.
The house was built in 1964, and we have been in it for 19 years. We had a new dual resin tank softener unit installed as soon as we took ownership. No leaking pipes so far. When we moved in, the original tub spigot for the main bathroom had a bunch of calcium deposits at the outlet. We remodeled that bathroom completely.
What would I need in order to check the copper content of the softened water? Is it time for me to send samples of my well, my softened well, and my RO off to Ward Labs? I'm retired and on fixed income.
Last edited:
By all means check the Primer but take note that since its original posting 8 years ago the community has pretty much decided that it is happier with mineral levels about half those in No. 1 in that tread. That's why I recommended half a tsp (about 2.5 grams) per 5 gal. in No. 27 here.
If you want to but as I said in #43 your softener would have to be in a pretty sorry state to pass enough calcium to get you pHsat > pHsample. But you might want to check on its performance.Should I see if I can find the GH/KH test kit, and have a go at my softened water with it?
A copper test kit. https://www.amazon.com/dp/B0006JDWH8/?tag=skimlinks_replacement-20What would I need in order to check the copper content of the softened water?
I thought I was too until the end of last week!...on fixed income.
Last edited by a moderator:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I thought I was too until the end of last week!
Thanks for the link to the copper test kit.
Did you find a job?
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I should have mentioned that just post the softener is some sort of oxidizer or oxygenator unit. It looks a lot like a single resin bed cylinder from our dual cylinder softener, only thinner and taller. I have no idea what this oxygenating? cylinder unit is for or does.
Edit: I just found an oxidation unit that looks a lot like ours on the net, and the blurb states that it is for the removal of H2S, Iron, and Manganese.
I also recall the softener/oxidizer installer stating 19 years ago that our well water has about 38 grains of hardness. I believe the cylinders for the softener switch out every 250 or 300 gallons. One is on line, while the other is regenerating....
Edit: I just found an oxidation unit that looks a lot like ours on the net, and the blurb states that it is for the removal of H2S, Iron, and Manganese.
I also recall the softener/oxidizer installer stating 19 years ago that our well water has about 38 grains of hardness. I believe the cylinders for the softener switch out every 250 or 300 gallons. One is on line, while the other is regenerating....
Last edited:
It oxidizes S- to S which gets trapped on the medium and backwashed away. It also oxidizes Fe(II) to Fe(III) which forms Fe2(OH)3 gel which gets caught on the medium and back-washed away.
A job? In today's crazy PC world I wouldn't make it through the first day!
A job? In today's crazy PC world I wouldn't make it through the first day!
Similar threads
- Replies
- 12
- Views
- 667
- Replies
- 17
- Views
- 1K
Latest posts
-
-
-
-
-
-
Need help diagnosing lower than expected efficiency
- Latest: Brockness Monster
-












































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)

