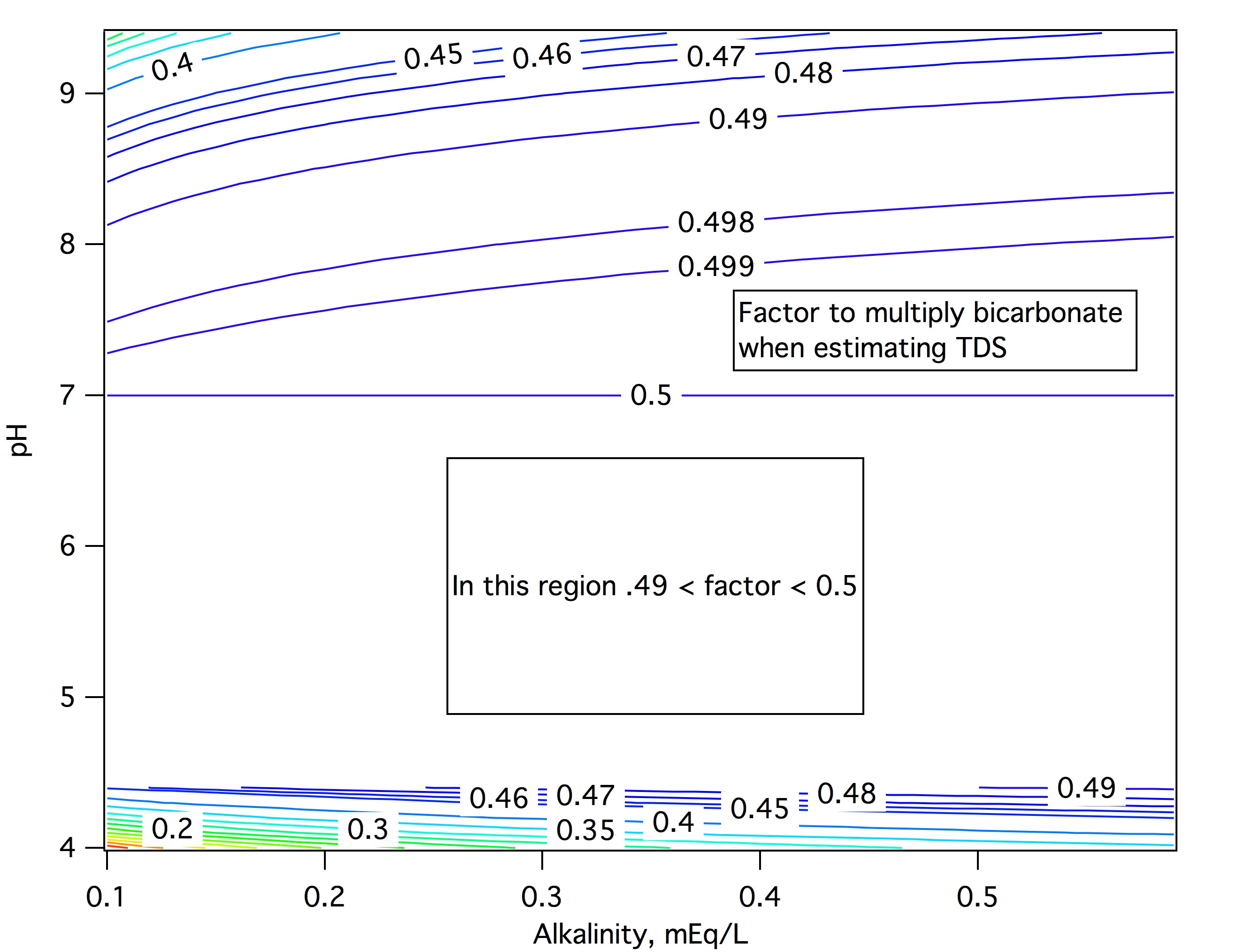
Interestingly enough, if one is to sum the ionic constituents, one must sum only 50% of the HCO3- if expecting to achieve a degree of validity via this method.
I had never thought of that nuance (always sloppily thinking of TDS as representing Total Dissolved Stuff) but indeed the statement is true.
[Edit]: What followed here was a graph showing how much carbon dioxide escapes when a calcium bicarbonate solution is evaporated as a fraction of the bicarbonate but that's not what we are talking about. We don't care about the dissolved CO2 that stays as a gas that leaves. We are concerned about the part that became bicarbonate. [End Edit]
Here's how it works (this is the Brewing Science Formum): x moles of calcium carbonate is dissolved by y moles of carbonic acid derived from y moles of carbon dioxide and y moles of water. The y moles of CO2 represent the Total Dissolved Gasses (TDG) which, of course, they really don't as we don't enter the masses of dissolved nitrogen, CO2, argon etc. In any case charges must balance. From x moles of CaCO3 we get 2*x positive charges from the calcium ions and x*Qc negative charges from the bicarbonate ions that are formed from the x moles of CaCO3. Qc is the charge on 1 mole of carbo, is a negative number and is a function of pH. The y moles of dissolved CO2 lend further negative charge of y*Qc. Thus 2*x + x*Qc + y*Qc = 0 as charge must balance.
The total carbo is Ct = x + y. Thus we have two equations we can easily solve for y, the amount of dissolved CO2:
y = Ct/( 1 - (Qc/(2 + Qc)))
[Edit] The part of y that leaves as dissolved but unconverted (to bicarbonate) is f0*Ct (fi is the fraction of Ct that carries charge -i) so the amount of CO2 leaving that comes from the bicarbonate is y - f0*Ct. The bicarbonate in the solution if f1*Ct so the fraction of this that goes out as CO2 is (y - f0)/f1.
For convenience set Ct = 1. Then y = 1/(1-Qc/(2+Qc)) = 1/(2/(2 + Qc) =1 - Q/2 = 1 - f1/2 - f2. Then y -f0 = 1 - f0 - f1/2 - f2. But f0 + f1 + f2 =1 implies that 1 - f0 - f2 = f1 so that y - f0 = f1 - f1/2 = f1/2 and dividing by f1 gives (y - f0)/f1 = 0.5. Exactly! The only thing we have neglected here is the charge on the water itself which will have an effect.
In the first equation we should have
2*x + x*Qc + y*Qc + Qw = 0 as charge must balance. The solution for y becomes
y = ( Ct + Qw/(2 + Q)) / ( 1 - (Qc/(2 + Qc))) and we can proceed as before except that it is clear that the effect of Qw is going to depend on the total carbo in the water. For large amounts of carbo it will dominate and the factor will be close to 0.5. For weaker (less alkaline) solutions the effect will be stronger at high pH. I'll look into this further and post more if this surmise leads to a significant result.





































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)