DuncB
Well-Known Member
I'm making an English Barleywine tomorrow ( Thomas Hardy ale ).
I add all of my salts to the Mash for the barley wine and no sparge for this. Previously I just have acidified the sparge water and treated for chlorine, but I've always wondered whether I should add some salts as well.
Some salts will be in the grains from the first treatment for the main brew but will enough get to the final runoff and beer?
My base water is
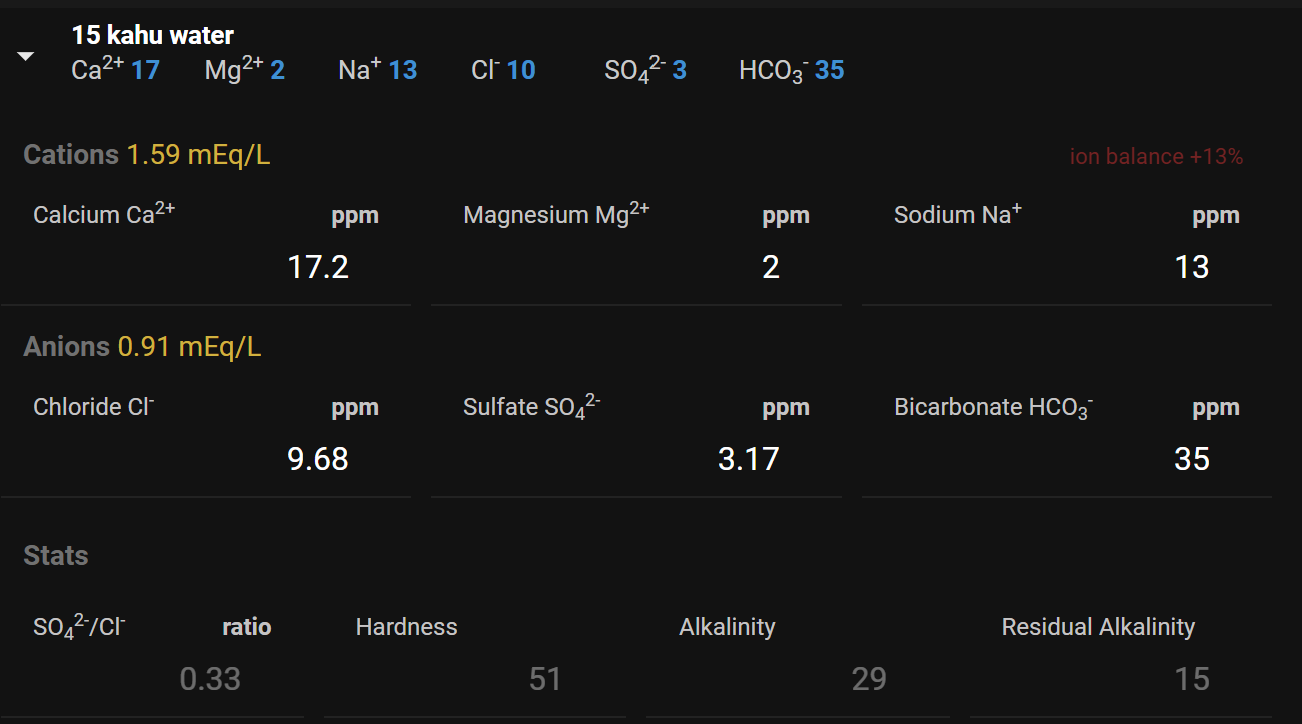
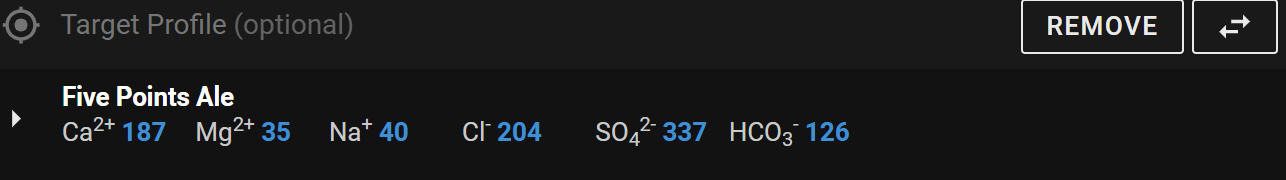
Barley Wine Water target
Thanks
I add all of my salts to the Mash for the barley wine and no sparge for this. Previously I just have acidified the sparge water and treated for chlorine, but I've always wondered whether I should add some salts as well.
Some salts will be in the grains from the first treatment for the main brew but will enough get to the final runoff and beer?
My base water is

Barley Wine Water target

Thanks
































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)



























