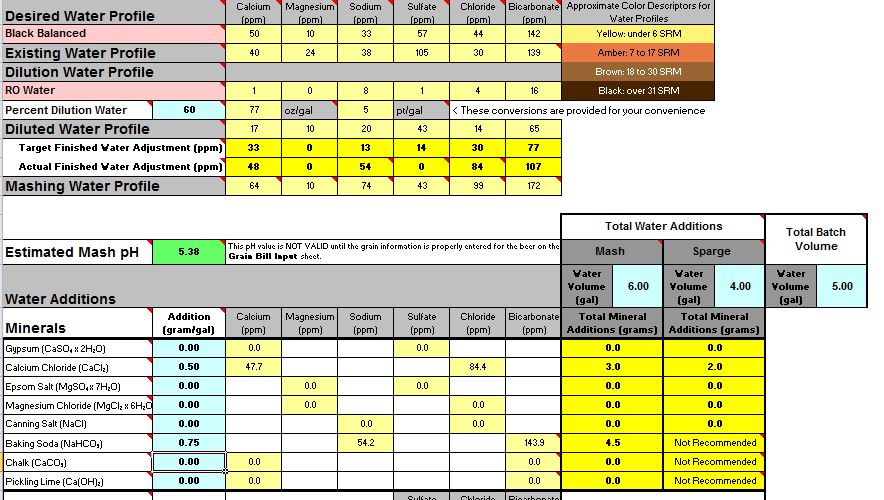
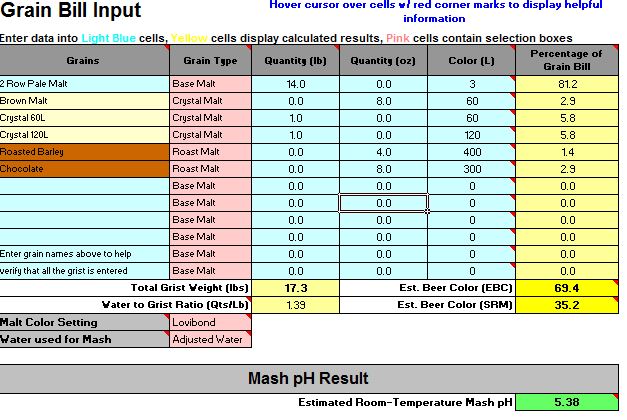
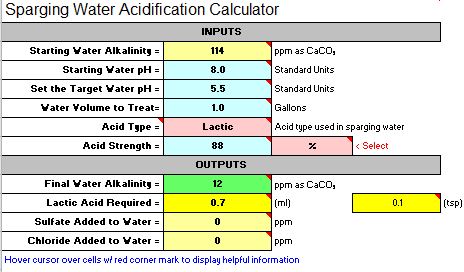
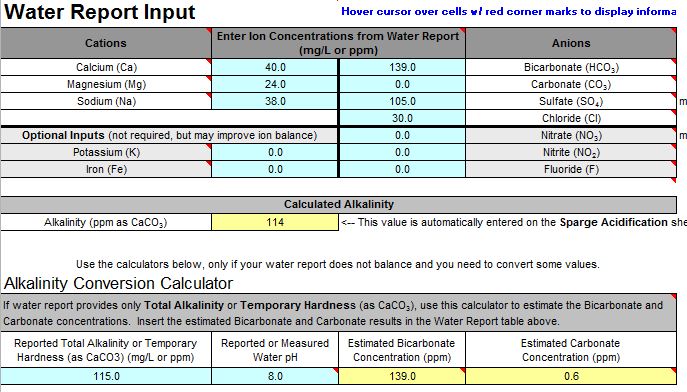
It might well have. 6.6 is pretty high. The beer will probably not be ruined by this. In fact it may well be quite enjoyable. After all, home brewers have been making decent beer since well before pH even entered our lexicon. It will definitely not be as good, however, as it could have been.
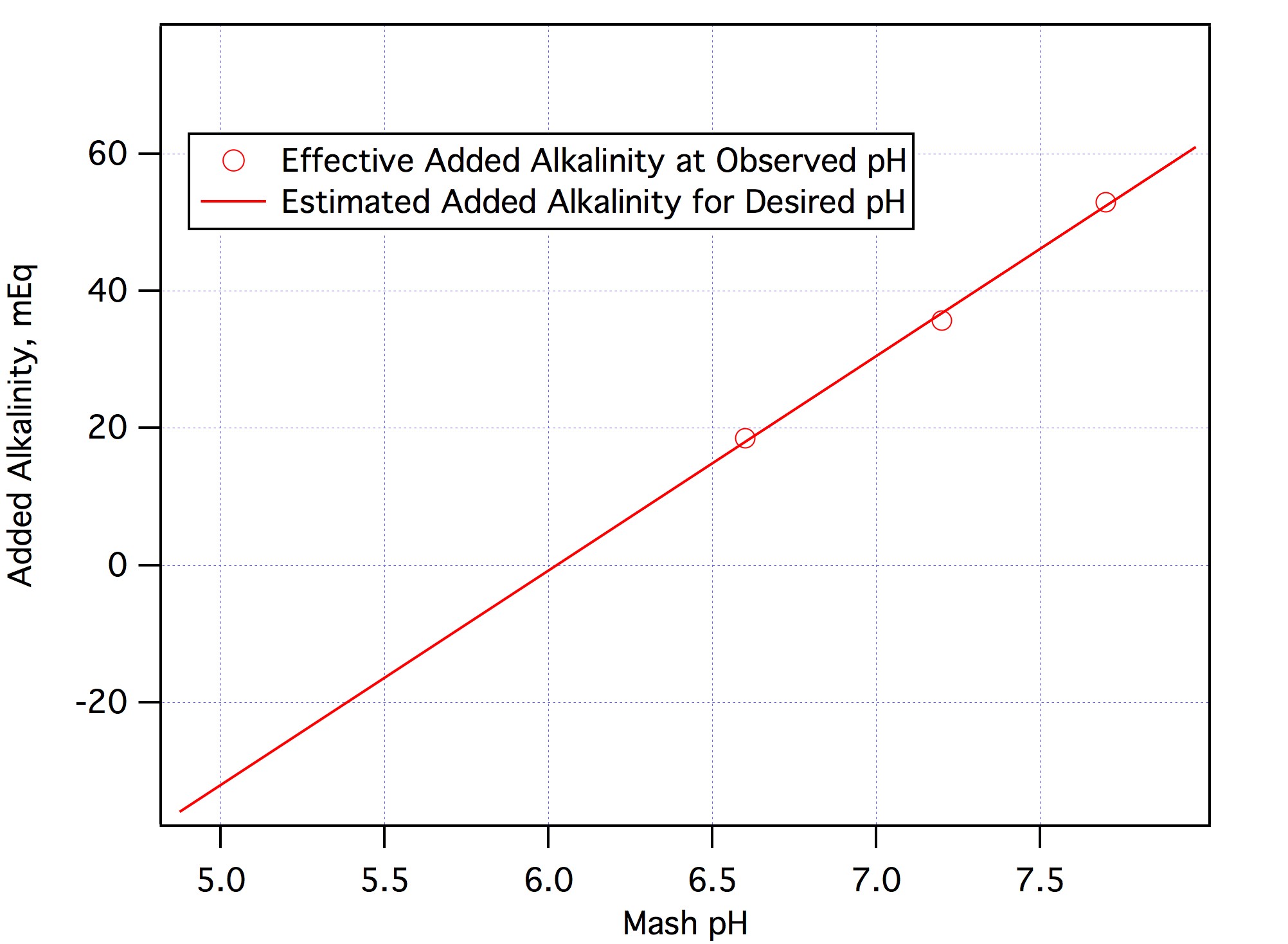
In No. 2 I said that I was guessing that the problem was adding the baking soda. If we want to apply a little science to the problem (which is why I assume you came to the Brewing Science forum) we can test that guess by looking at the data you collected. The 4.5 grams of baking soda contribute about 4500/124 = 53 mEq of alkalinity as 124 is the molecular weight of NaHCO3. With that much added alkalinity you got a pH of 7.7. The normality of 88% lactic acid is about 11.5 mEq/mL i.e. each mL of it can neutralize 11.5 mEq of alkalinity. Thus the effective added alkalinity with 4.5 grams of baking soda and 1.5 mL of lactic acid is 53 - 1.5*11.5 = 35.75 mEq. This gave you a pH of 6.6. Adding another 1.5 mEq of acid would cancel another 17.25 mEq of the bicarb and give you an effective added alkalinity of 53 - 1.5*11.5 - 1.5*11.5 = 18.5 mEq. This gave you a pH of 6.6. Plotting those three points gives the three open circles on this graph:
View attachment 551570
Fitting a straight line through those points shows that the relationship between added alkalinity and mash pH for this mash is pretty linear so that extrapolating the fit line to the region of desired pH ought to give an indication as to how much alkalinity you should have added for any desired pH. Suppose you wanted 5.5. The graph shows that you should have added approximately -16.4 mEq of alkalinity (the fit line is mEq = -188.405 + 31.2775*pH). That is +16.4 mEq acid. With lactic's normality of 11.5 that's 18/11.5 = 1.43 mL.
Thus we confirm the suspicion that you should have added less bicarbonate. In fact it appears that you should have added acid instead of bicarbonate. This happens fairly frequently with spreadsheets that have crude models of colored malts based often only on their color (°L) or type. Of course there is also the possibility that you entered data into your spreadsheet in error.
Now this does not guarantee that you can just go out next time and add 1.43 mL lactic acid and no bicarbonate to a grist of the same composition and hit pH 5.5. You may have the same malts but from a different maltster or from the same maltster but a different batch etc. and/or your water's alkalinity may be subject to variation over time. And, while titrating malts or mashes as you effectively have done here is indeed the way to accurately control mash pH that wasn't your intention and so your observations cannot be considered the robust measurements that we would expect if it were your intention. Furthermore, extrapolation outside the range of observation is what we call "bold" extrapolation. The line probably continues as a more or less straight line but we as we do know that malt titration curves are non linear (if not terribly so) we are on slightly shaky ground at pH 5.5.
What it does strongly suggest, however, is that a test mash with 1.43 mL of lactic acid (scaled to the size of the test mash, of course) would be a good place to start.









































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)