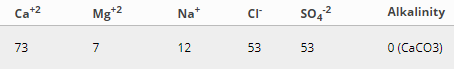
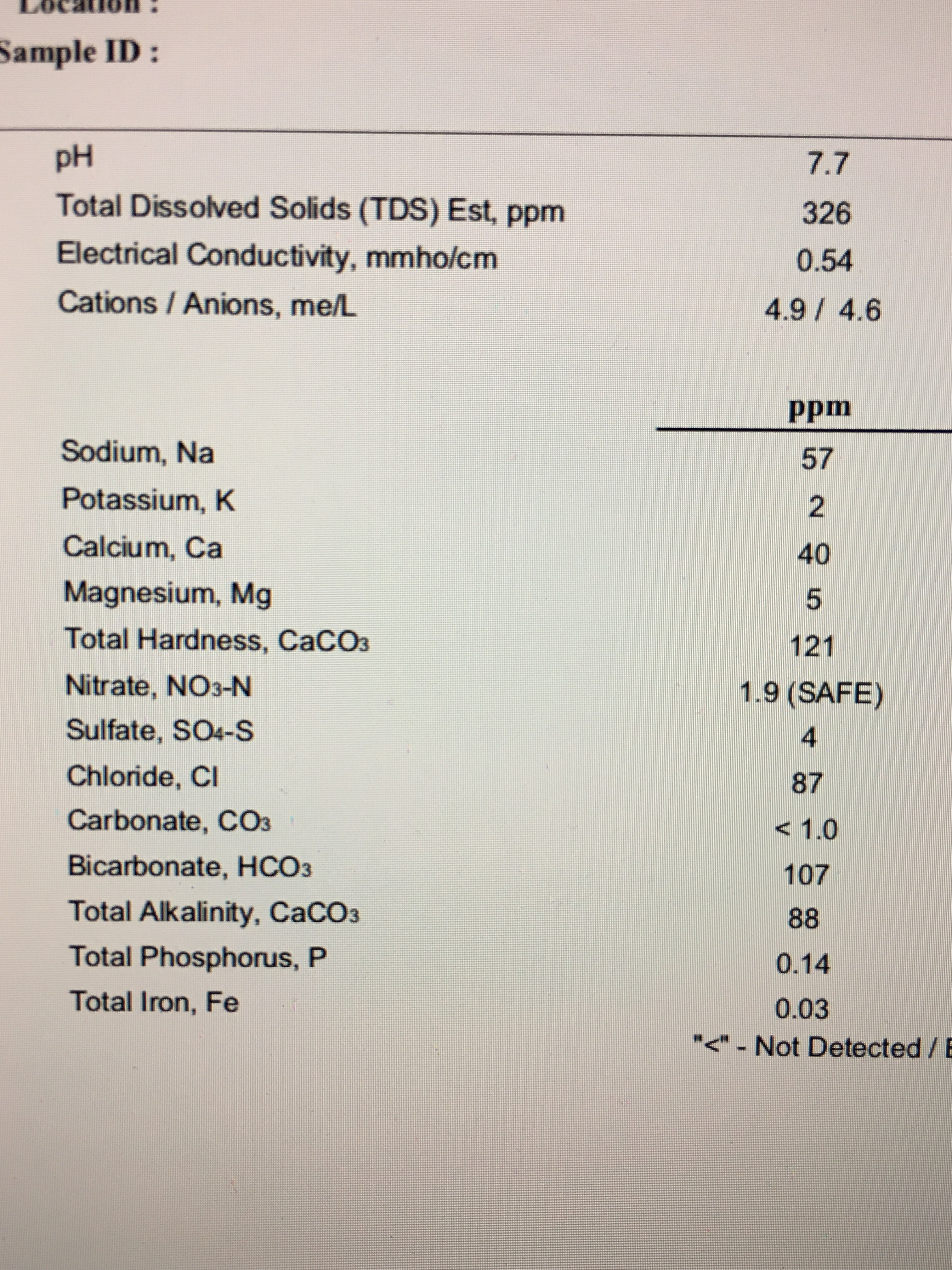
I'm still trying to figure out what the deal is with alkalinity/hardness/carbonate.
Hardness as CaCO3: High levels Mg and Ca both make water 'hard'. Hard water doesn't lather, doesn't quite make you feel clean when you wash with it and leaves behind scale. If all of the Mg and Ca in your water was present as Calcium carbonate (CaCO3), how much CaCO3 would there be? That's the number that is reported for hardness as CaCO3. There might not even be any Carbonate in the water (the Calcium and/or Magnesium could have come from Chlorides or Sulphates). It's not really useful in brewing - we generally only want the values for Ca and Mg on their own.
Alkalinitiy as CaCO3: Alkalinity is important because it tells us how much the water can resist downward change in pH. Two waters can both have the same pH, but one with high alkalinity will stay close to that pH if a small amount of acid is added, whilst one with low alkalinity will have it's pH significantly lowered. Calcium carbonate is the main contributor to natural alkalinity, which will increase a water's bicarbonate. If ALL of the alkalinity in the water was from Calcium carbonate (CaCO3), how much CaCO3 would there be? That's the number that is reported as alkalinity as CaCO3. Divide by 1.2 to get bicarbonate.
Carbonate: Not generally important from a water report. Bicarbonate or Alkalinity as CaCO3 is what we need.
Bicarbonate: The main ion that makes up natural alkalinity in water. It is approximately 1.2 times the Alkalinity as CaCO3. You only need to know either Alkalinity OR Bicarbonate for brewing.
Bicarbonate around the 100ppm range is easy to counter with acid additions even for pale beers. Much above this, things get trickier in pale beers. Along with the acid needed to counter the alkalinity, you are adding an associated anion (eg. lactate with lactic acid; phosphate with phosphoric acid; chloride with hydrochloric acid). If alkalinity is high, the amount of acid needed is high, so the anion concentration becomes high and might negatively affect the beer. Around the 200ppm range you need to think about blending acids and/or boiling water to remove bicarbonate and/or brew darker beer and/or blend with RO water. Up around the 300ppm+ range, most brewers would just use RO water (or stick with stouts and porters).
This is how I understand it and some is my opinion, and is probably not 100% correct (simplifications rarely are), but hopefully helps with a not too technical explanation.









![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)