Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
1st priority: Someone please check my work as seen below for mistakes.
Required items list:
1N HCl (1 Normal Hydrochloric Acid)
Bromocresol Purple indicator solution
10 mL Graduated Cylinder
50 mL Graduated Cylinder
100 mL Beaker
Eye Dropper or Pipet
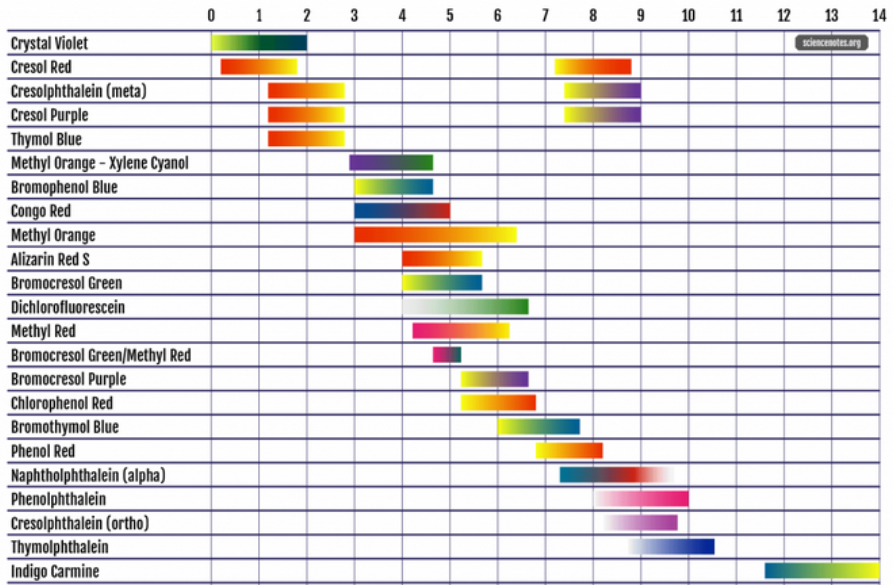
Colors Of Bromocresol Purple indicator in water:
----------------------------------------------------------------
~pH 6.3 and above = purple
~pH 6.0 = violet
~pH 5.6 = violet/yellow
~pH 5.4 = yellow/violet
~pH 5.2 and below = yellow
Process Steps:
--------------------
1) Calibrate the delivery of your chosen eye-dropper or pipet: Add drops of 1N HCl Acid from your eye-dropper or pipet into a clean and dry 10 mL Graduated Cylinder until you reach 5 mL, and record the number of drops required to reach 5 mL.
2) Divide answer from #1 by 5. Record the 'number of drops divided by 5' result as your Eye-Dropper or Pipet's "Drops per mEq"
(Whereby: 1.0 mL of 1N Acid = 1 mEq of acid)
3) Measure out exactly 50 mL of your water to be tested for Alkalinity within a 50 mL Graduated Cylinder, then transfer this 50 mL of water into a 100 mL beaker.
(Note: 50 mL = 0.050 Liters = 1/20 of a Liter as your sample size)
4) Add a few drops of Bromocresol Purple indicator to your water and gently swirl (your water should initially become violet to most likely purple)
5) Add to your water individual drops of 1N HCl Acid via your "Drops per mEq" calibrated eye-dropper or pipet, and gently swirl after each drop until you achieve a transition in color to violet/yellow or yellow/violet. Record the counted number of drops of 1N HCl this required. Your water should now be at about pH 5.4-5.6.
6) Determine the mEq's of acid delivered:
mEq's of acid delivered = # of drops from step #5 divided by "Drops per mEq" from step #2.
7) Multiply the line #6 determined mEq's by 20 to determine the mEq's/L (mEq's per Liter) of Alkalinity titrated for your water.
8) Multiply the line #7 result by 50 to convert mEq's into the ppm (mg/L) of Alkalinity removed from your water via acid titration whereby to reach pH 5.4-5.6.
(Note: Multiply by 50.04345 instead of 50 if you are highly persnickety)
9) Divide the line #8 result by 0.87 if you stopped titrating (adding acid drops) at ~violet/yellow (~pH 5.6). Or divide the line #8 result by 0.89 if you stopped titrating at ~yellow/violet (~pH 5.4).
The #9 Result = "Total Alkalinity (as CaCO3)" in ppm (or mg/L) for your water.
Required items list:
1N HCl (1 Normal Hydrochloric Acid)
Bromocresol Purple indicator solution
10 mL Graduated Cylinder
50 mL Graduated Cylinder
100 mL Beaker
Eye Dropper or Pipet
Colors Of Bromocresol Purple indicator in water:
----------------------------------------------------------------
~pH 6.3 and above = purple
~pH 6.0 = violet
~pH 5.6 = violet/yellow
~pH 5.4 = yellow/violet
~pH 5.2 and below = yellow
Process Steps:
--------------------
1) Calibrate the delivery of your chosen eye-dropper or pipet: Add drops of 1N HCl Acid from your eye-dropper or pipet into a clean and dry 10 mL Graduated Cylinder until you reach 5 mL, and record the number of drops required to reach 5 mL.
2) Divide answer from #1 by 5. Record the 'number of drops divided by 5' result as your Eye-Dropper or Pipet's "Drops per mEq"
(Whereby: 1.0 mL of 1N Acid = 1 mEq of acid)
3) Measure out exactly 50 mL of your water to be tested for Alkalinity within a 50 mL Graduated Cylinder, then transfer this 50 mL of water into a 100 mL beaker.
(Note: 50 mL = 0.050 Liters = 1/20 of a Liter as your sample size)
4) Add a few drops of Bromocresol Purple indicator to your water and gently swirl (your water should initially become violet to most likely purple)
5) Add to your water individual drops of 1N HCl Acid via your "Drops per mEq" calibrated eye-dropper or pipet, and gently swirl after each drop until you achieve a transition in color to violet/yellow or yellow/violet. Record the counted number of drops of 1N HCl this required. Your water should now be at about pH 5.4-5.6.
6) Determine the mEq's of acid delivered:
mEq's of acid delivered = # of drops from step #5 divided by "Drops per mEq" from step #2.
7) Multiply the line #6 determined mEq's by 20 to determine the mEq's/L (mEq's per Liter) of Alkalinity titrated for your water.
8) Multiply the line #7 result by 50 to convert mEq's into the ppm (mg/L) of Alkalinity removed from your water via acid titration whereby to reach pH 5.4-5.6.
(Note: Multiply by 50.04345 instead of 50 if you are highly persnickety)
9) Divide the line #8 result by 0.87 if you stopped titrating (adding acid drops) at ~violet/yellow (~pH 5.6). Or divide the line #8 result by 0.89 if you stopped titrating at ~yellow/violet (~pH 5.4).
The #9 Result = "Total Alkalinity (as CaCO3)" in ppm (or mg/L) for your water.
Last edited:















































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)