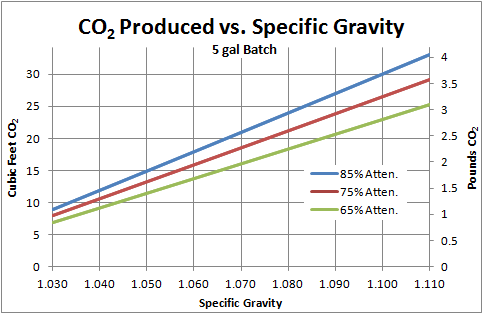
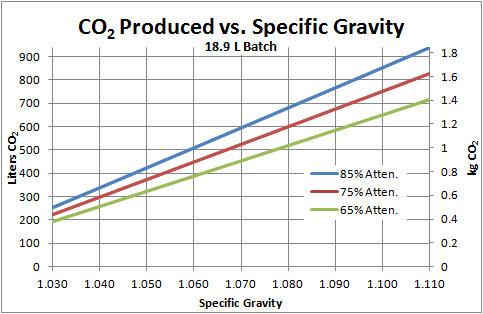
Just wondering if anyone was able to get an estimate on how much co2 is given off from your normal 5 gal batch. I remember reading some years ago about someone weighing their beer as it ferments and 4lbs of co2 is what seemed to be the magic number.
I have a temp controlled fermentation freezer vented into a 4x4 grow tent. Every time I open the tent I can smell the fermentation and figure their must at least be some benefit to the plants.
Just wondering if anyone has tried anything similar before?

I have a temp controlled fermentation freezer vented into a 4x4 grow tent. Every time I open the tent I can smell the fermentation and figure their must at least be some benefit to the plants.
Just wondering if anyone has tried anything similar before?