Thorpe
Well-Known Member
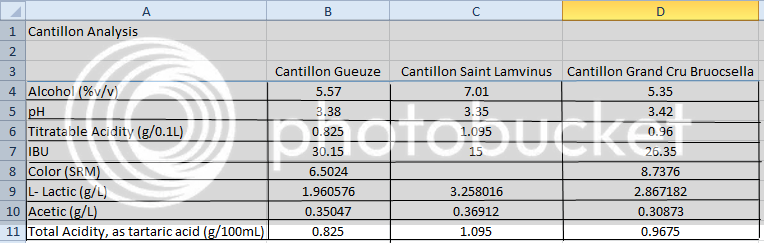
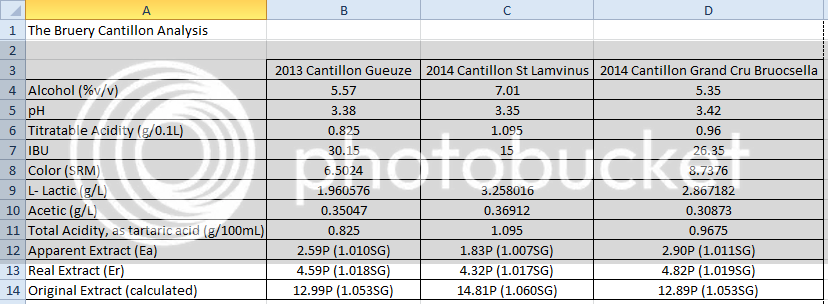
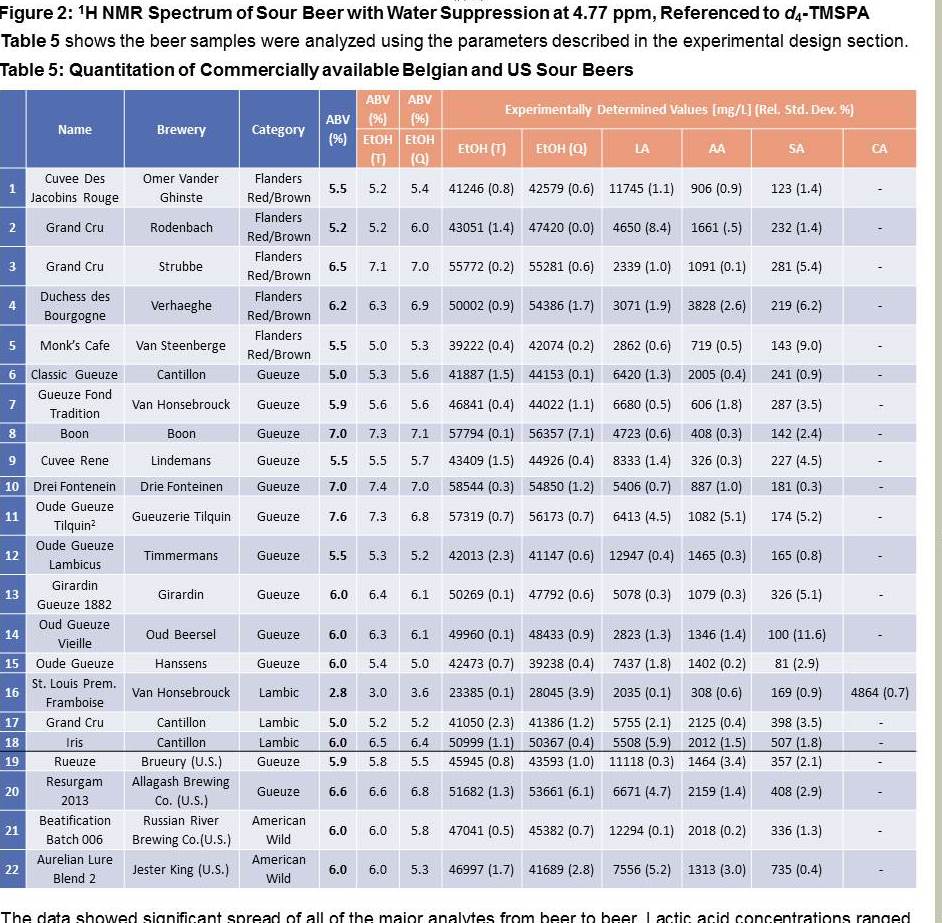
Absolutely! Existing literature shows a fair bit of ABV variation between barrels of spontaneously fermented beer. What's strange is that these two beers have the same OG, the same FG, but a different ABV. Most people treat this is a simple three-variable system: if you know two of the variables {OG, FG, ABV}, then the third one is fixed. Obviously reality can be more complicated, but for most fermentations the three-variable simplification works really well. Given that alcohol makes up a large mole fraction of the finished product and has a markedly different density than water, SourBrewer's results indicate that there must be some other fermentation product whose concentration varies considerably between the two beers and has a markedly different density as well. In essence, a hidden fourth variable.
Couldn't the answer be that some of the organisms that get into the wort are converting sugar into something other than CO2 and alcohol?