I've been scouring the forums for advice on building a hefeweizen water profile. Fortunately my water is low-mineral, so I can build what I like. Everything is < 8 PPM except bicarb which is 24 PPM.
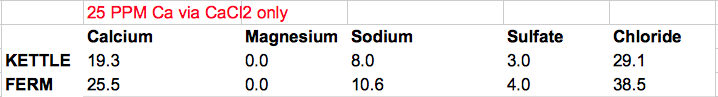
@mabrungard's advice in another thread was to keep the water as low mineral as possible while getting Ca to ~ 50 PPM via CaCl2 only, and to "live with" the amount of Cl this added.
In my case this plan yields ~50 PPM Ca and ~80 PPM Cl in the finished product. (see below)
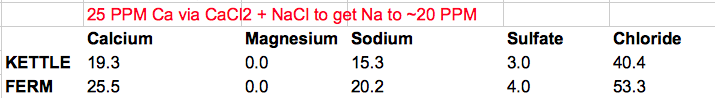
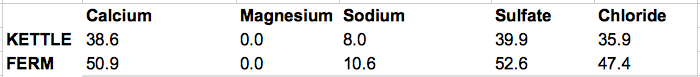
Does this look reasonable? I've never made a hefeweizen with ~80 PPM chloride in the glass. I could try to use some gypsum as another Ca source, and suffer some sulfate as the price of lowering chloride. In that scenario, Ca, Cl, and SO4 are all ~50 PPM in the finished product. (details also below)
Anyone have an opinion on which approach will taste better?
Other details that might be useful: I am using 6.7% CaCl2 solution, and a single-vessel eBIAB system with a starting water volume of 8.9 gal. I'm targeting a mash pH of 5.25 via use of phosphoric acid. Grain bill is ~70% red wheat and ~30% pilsen.
Thanks for the input!

@mabrungard's advice in another thread was to keep the water as low mineral as possible while getting Ca to ~ 50 PPM via CaCl2 only, and to "live with" the amount of Cl this added.
In my case this plan yields ~50 PPM Ca and ~80 PPM Cl in the finished product. (see below)
Does this look reasonable? I've never made a hefeweizen with ~80 PPM chloride in the glass. I could try to use some gypsum as another Ca source, and suffer some sulfate as the price of lowering chloride. In that scenario, Ca, Cl, and SO4 are all ~50 PPM in the finished product. (details also below)
Anyone have an opinion on which approach will taste better?
Other details that might be useful: I am using 6.7% CaCl2 solution, and a single-vessel eBIAB system with a starting water volume of 8.9 gal. I'm targeting a mash pH of 5.25 via use of phosphoric acid. Grain bill is ~70% red wheat and ~30% pilsen.
Thanks for the input!









![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)