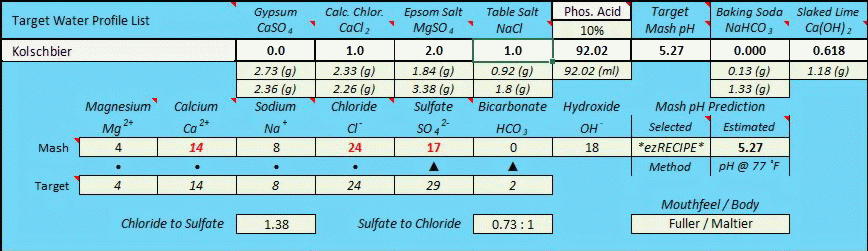
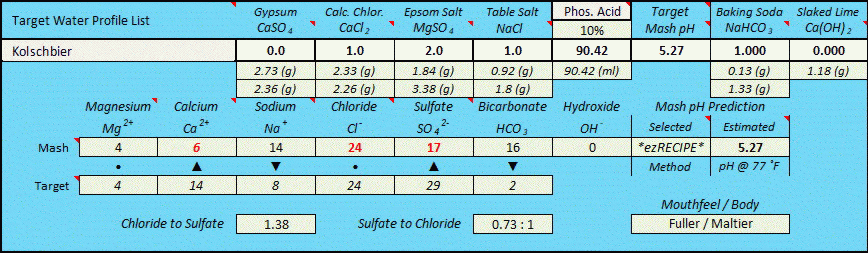
After having concluded the fixed 0.618:1 ratio (as per my infinite regression determination method seen above) I subsequently began to look at potentially critical pKa related ion dissociation factors, whereby baking soda is an extremely weak base with only partial dissociation at near mash pH due to its pKa1 value of 6.35 (which means that it is only 50% as basic at pH 6.35 as one would initially presume when using rudimentary level chemistry methods based solely upon stoichiometric equation balancing as I had initially been doing), and slaked lime is an extremely strong base with much higher levels of dissociation completeness at typical mash pH's, nearing complete dissociation, but not quite. If all else was equal between NaHCO3 and Ca(OH)2 the 0.618:1 ratio would clearly be true (and it may yet prove to work out to be that way), but in the real world all is likely not equal between them due to the vast differences in ion dissociation witnessed between them at typical mash pH's. Only actual "real world" 'side by side' testing of identical mashes utilizing both bases to raise pH will finally resolve this across the spectrum of pH's that are typically targeted, but for now I am cautiously presuming that it likely takes measurably less Ca(OH)2 than the "perfect world" work I did above would indicate, and that the equivalence ratio is highly pH target dependent and therefore not of a fixed value.
But it gets even more complex than settling upon a strictly pKa based solution, such as AJ deLange seems to lean toward in his math models (albeit that I do not want to, nor do I have any right to presume to speak for him, such that I'm not worthy to tie his shoes), since pKa related chemical equation reversal is clearly broken when one of the stoichiometry components of the "balanced" chemical equation evolves out of the solution mix (the developing wort in this case) as a gas, and is thereby no longer present in solution whereby to drive pKa associated equation reversal. For this case the breaks placed upon idealized reaction completeness via pKa related weak dissociation are somewhat lifted, and completion is more closely (and perhaps even fully) approached. But even gasses have solubilities and thereby do not completely evolve away....
I wish AJ was still actively participating on this forum, as his input would be much valued in assisting to resolve this. But the short answer is that the equivalency relationship of Ca(OH)2 to NaHCO3 is likely to be far more complex than an across the board presumption of either a fixed 0.618 to 1 ratio (due to the downward pH impact of the Ca++ ion within Ca(OH)2), or a 0.441 : 1 ratio based purely upon idealized stoichiomitry while ignoring any Ca++ impact, or a result based strictly upon dissociation constants (pKa or Ka), or a result based upon dissociation constants plus the Ca++ factor....