When I get too many things in my brain all at once it freezes up. Forgive the pictures but supposedly they're worth a thousand words and I'm already long-winded enough.
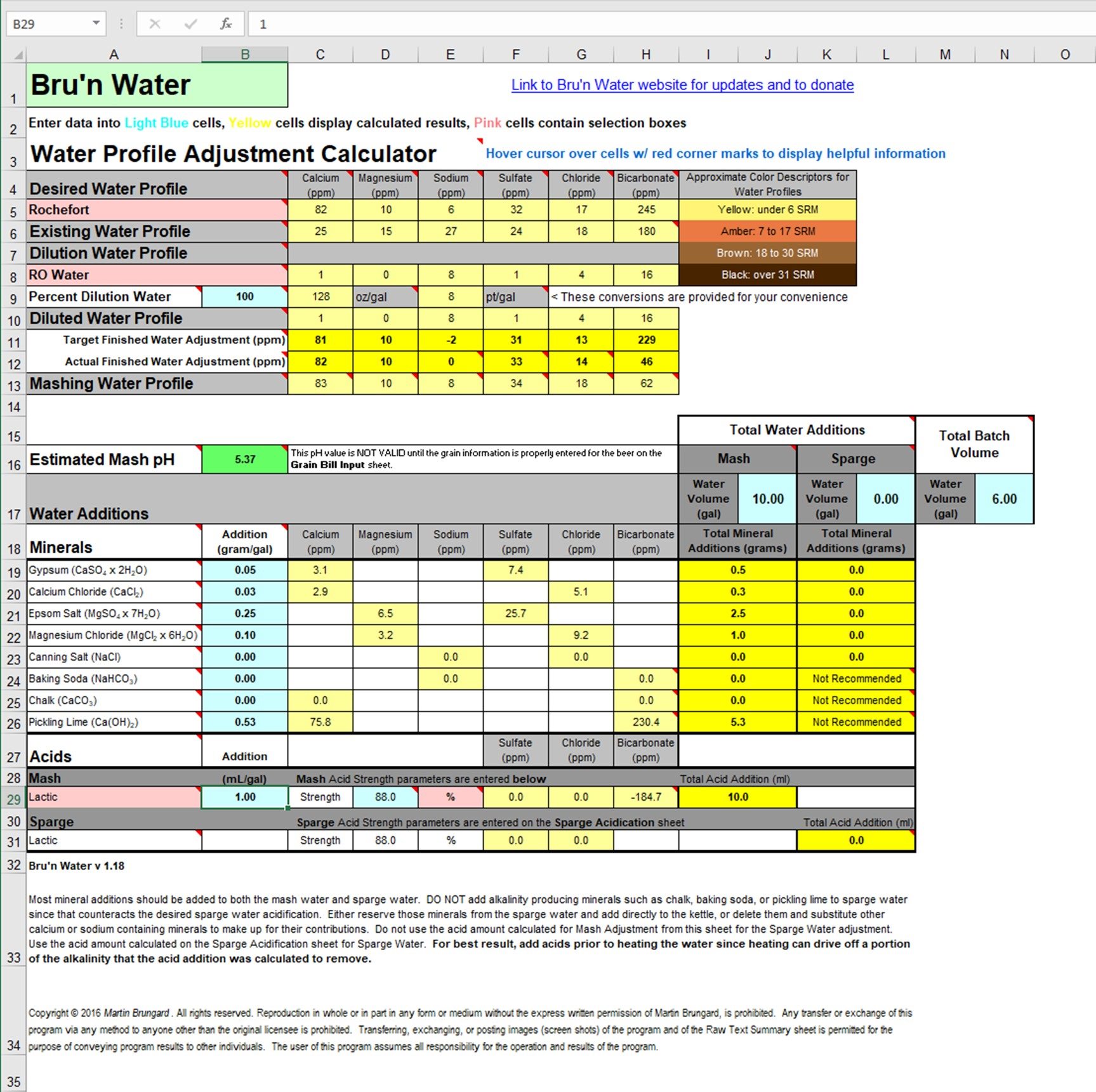
I wrote "No sparge" in the title, but I am going to sparge. What my question is really about is adjusting the mash and sparge water separately or just adjusting all the water the same. The first picture is how the numbers worked out adjusting all the water the same. I know adding pickling lime and lactid acid is not recommended, but I wanted to get the numbers as close to the Rochefort profile as possible and get the pH right.
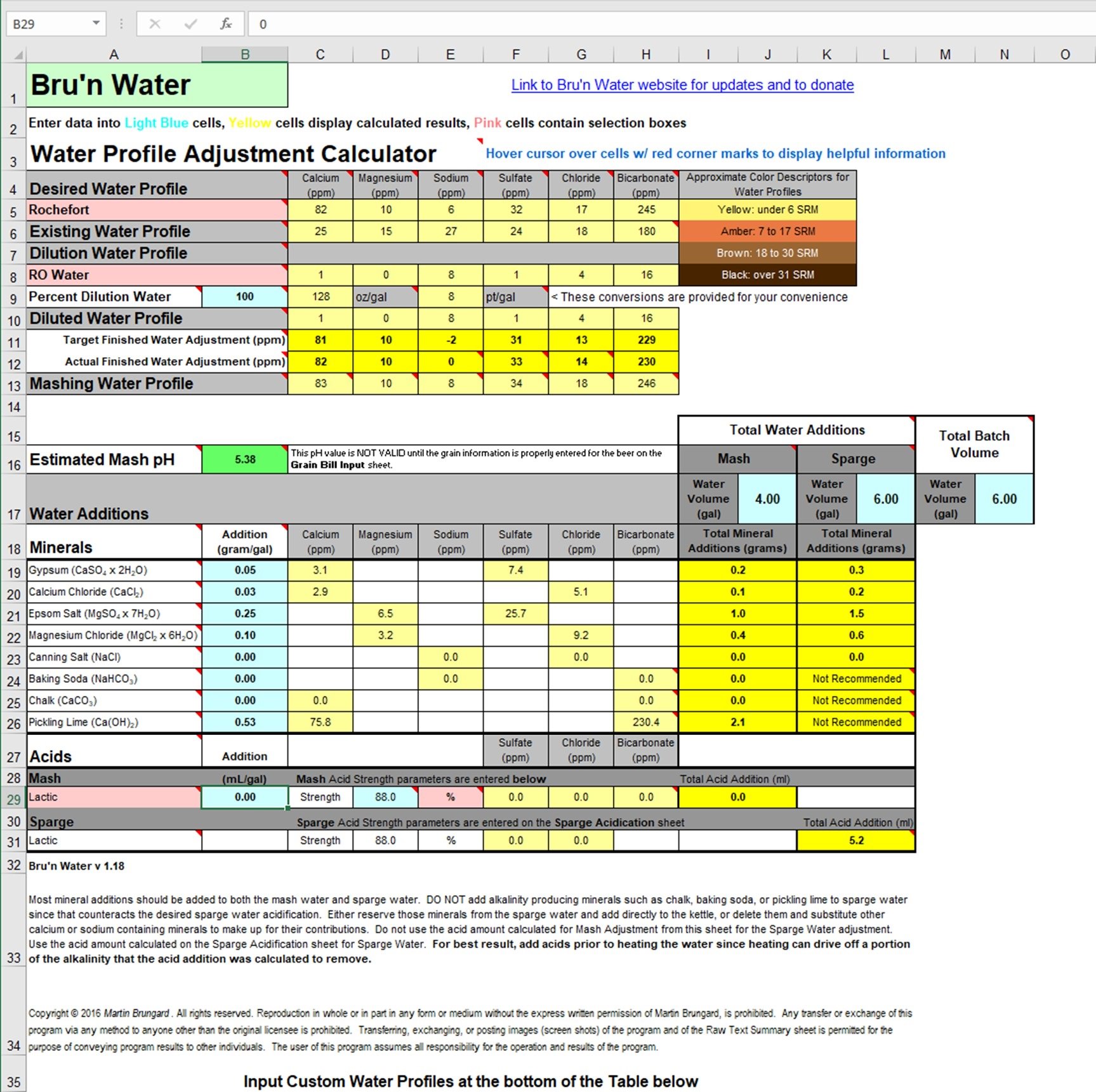
The second picture is adjusting the mash and sparge water separately. All of the other additions are the same except I don't need to add acid.
My question is which is the better way to go? I've always done the water additions a day or two before I brew to allow the minerals to absorb and the water to stabilize. Apparently I've had an over-simplified understanding of pH. I've read where you want to shoot for a pH of about 5.4-5.6 for making beer. I've always used Bru 'N Water and I've always tried to keep the pH in that range. Sometimes I check the water with my pH meter, sometimes I get lazy. I checked the pH of the mash after the grains were added a couple times but wasn't real sure what I was looking for so it was kind of a waste of time really. And finally, I was watching a video the other day where the guy was talking about a beer he brewed and how the pH changed over the months he had it sitting and how the flavor changed as a result of the pH changing???
So apparently my understanding that the "water" should be 5.4 to 5.6 before I do anything else is a little naive. If I adjust the water separately for the mash and sparge I don't need to add acid, but then obviously the pH is going to be a lot higher when I add the sparge water. Which tells me that the pH of the sweet wort is probably important, and then the pH is going to change after it's boiled, correct? So is that pH as important? And every time I add hops or something else to the wort I'm going to change the pH. Is it important to check that every time and is there a number I should be looking for?
I wrote "No sparge" in the title, but I am going to sparge. What my question is really about is adjusting the mash and sparge water separately or just adjusting all the water the same. The first picture is how the numbers worked out adjusting all the water the same. I know adding pickling lime and lactid acid is not recommended, but I wanted to get the numbers as close to the Rochefort profile as possible and get the pH right.

The second picture is adjusting the mash and sparge water separately. All of the other additions are the same except I don't need to add acid.

My question is which is the better way to go? I've always done the water additions a day or two before I brew to allow the minerals to absorb and the water to stabilize. Apparently I've had an over-simplified understanding of pH. I've read where you want to shoot for a pH of about 5.4-5.6 for making beer. I've always used Bru 'N Water and I've always tried to keep the pH in that range. Sometimes I check the water with my pH meter, sometimes I get lazy. I checked the pH of the mash after the grains were added a couple times but wasn't real sure what I was looking for so it was kind of a waste of time really. And finally, I was watching a video the other day where the guy was talking about a beer he brewed and how the pH changed over the months he had it sitting and how the flavor changed as a result of the pH changing???
So apparently my understanding that the "water" should be 5.4 to 5.6 before I do anything else is a little naive. If I adjust the water separately for the mash and sparge I don't need to add acid, but then obviously the pH is going to be a lot higher when I add the sparge water. Which tells me that the pH of the sweet wort is probably important, and then the pH is going to change after it's boiled, correct? So is that pH as important? And every time I add hops or something else to the wort I'm going to change the pH. Is it important to check that every time and is there a number I should be looking for?


