This weekend I plan to brew an APA (Grain bill below) using 100% distilled water for the first time. Reason being is I have an annual golf outing in early May with friends from high school and I plan to bring a keg of this brew to the event. With that said I have noticed that the last 2 batches I brewed with my tap water seemed astringent. I have not sent my water in for analysis since 2 years ago so I am guessing it has changed. Since I do not have time to send a water sample in for analysis I am going to buy 100% distilled water.
Batch Size: 5.5 gallons
Batch Sparge
Grain Bill
8lbs 12oz - Pale 2 row (2L)
12oz - American Rye (3.7L)
12oz - Munich (10L)
4oz - Crystal 80L
4oz - White wheat (2.5L)
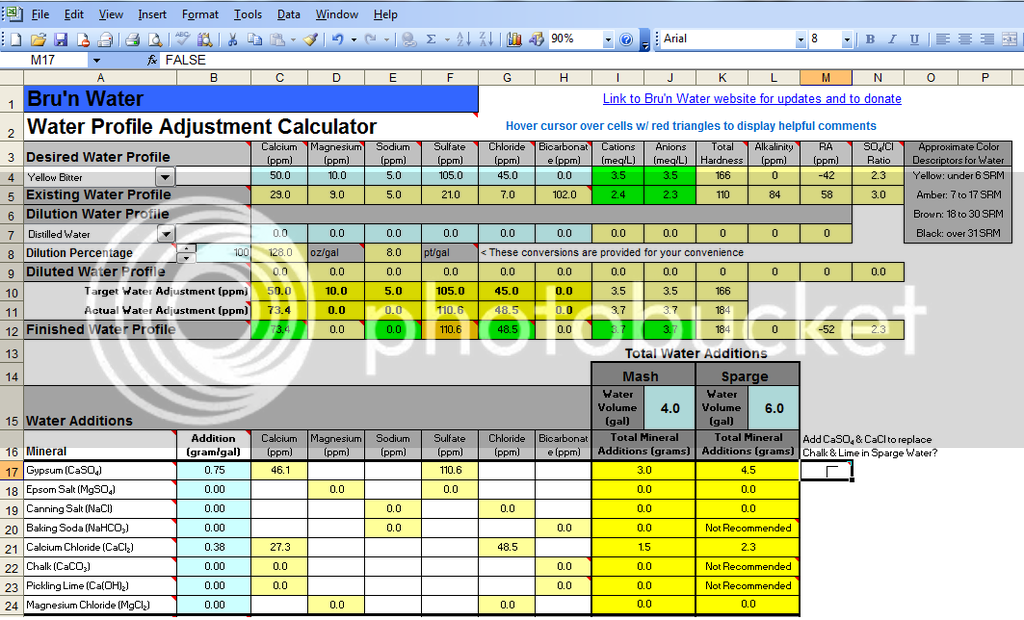
Attached is a screen shot of my finished APA profile with the minerals I added. Using these additions I get an estimated mash room temp pH of 5.4. Now I know the nominal pH of distilled water is 7 and I intend to add my Gypsum and CaCl mineral additions to my sparge water prior to starting my batch sparge. So my question is there a way to determine how much my sparge water pH will be reduced by my mineral additions using the software? I only have paper strips and a pH meter that reads pH at room temp. I could use my pH meter if I mix in my minerals to the sparge water at room temp but I am not sure if they will fully dissolve at this temp which could lead to an inaccurate reading.
Lets say that I receive an accurate pH reading from the room temp mineral additions and it has a pH of 6.5. Using the "sparge acifidication" tab I am trying to calculate how much latic acid I would need to reduce the pH down to 6. Is it safe to assume there is "0" alkalinity in my distilled water or should i use 1ppm for my latic acid calculation?
Maybe an easier way to accurately calculate my latic acid addition would be to add my "sparge" minerals directly to the boil kettle prior to boil. This would allow me to sparge with pure distilled water which would let me assume the starting pH of my sparge will be 7 and i can add 0.04mL of latic acid to reduce it to my desired pH of 6? See attached "APA sparge" picture
Thanks in advance for everyone's feedback!
Rob



Batch Size: 5.5 gallons
Batch Sparge
Grain Bill
8lbs 12oz - Pale 2 row (2L)
12oz - American Rye (3.7L)
12oz - Munich (10L)
4oz - Crystal 80L
4oz - White wheat (2.5L)
Attached is a screen shot of my finished APA profile with the minerals I added. Using these additions I get an estimated mash room temp pH of 5.4. Now I know the nominal pH of distilled water is 7 and I intend to add my Gypsum and CaCl mineral additions to my sparge water prior to starting my batch sparge. So my question is there a way to determine how much my sparge water pH will be reduced by my mineral additions using the software? I only have paper strips and a pH meter that reads pH at room temp. I could use my pH meter if I mix in my minerals to the sparge water at room temp but I am not sure if they will fully dissolve at this temp which could lead to an inaccurate reading.
Lets say that I receive an accurate pH reading from the room temp mineral additions and it has a pH of 6.5. Using the "sparge acifidication" tab I am trying to calculate how much latic acid I would need to reduce the pH down to 6. Is it safe to assume there is "0" alkalinity in my distilled water or should i use 1ppm for my latic acid calculation?
Maybe an easier way to accurately calculate my latic acid addition would be to add my "sparge" minerals directly to the boil kettle prior to boil. This would allow me to sparge with pure distilled water which would let me assume the starting pH of my sparge will be 7 and i can add 0.04mL of latic acid to reduce it to my desired pH of 6? See attached "APA sparge" picture
Thanks in advance for everyone's feedback!
Rob