markcurling
Well-Known Member
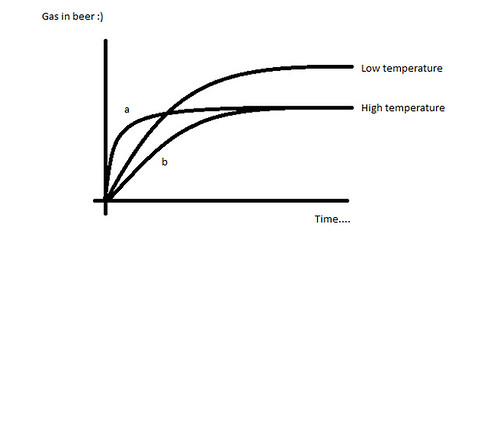
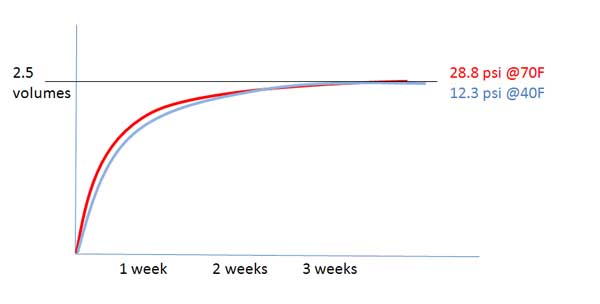
Gas only ever flows from high partial pressure to low partial pressure (i.e. towards equilibrium). Therefore CO2 will only flow into or out of the headspace in the direction of equilibrium. The CO2 is added by the yeast into the solution, which puts the CO2 in solution at a higher partial pressure than the headspace, forming a concentration gradient from the beer to the headspace.
Therefore I disagree with the wording where you say CO2 is "forced back in solution". This implies that the CO2 flows from solution, to headspace, to solution again. There can never be a time when the headspace has a higher partial pressure than the solution, so there can never be a net flow from the headspace to the beer. CO2 is added in solution, so the direction of flow can only ever be into the headspace. This is the exact opposite of force carbing, whereby you are adding CO2 to the headspace hence setting up a concentration gradient into the beer.
Therefore I disagree with the wording where you say CO2 is "forced back in solution". This implies that the CO2 flows from solution, to headspace, to solution again. There can never be a time when the headspace has a higher partial pressure than the solution, so there can never be a net flow from the headspace to the beer. CO2 is added in solution, so the direction of flow can only ever be into the headspace. This is the exact opposite of force carbing, whereby you are adding CO2 to the headspace hence setting up a concentration gradient into the beer.











































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)