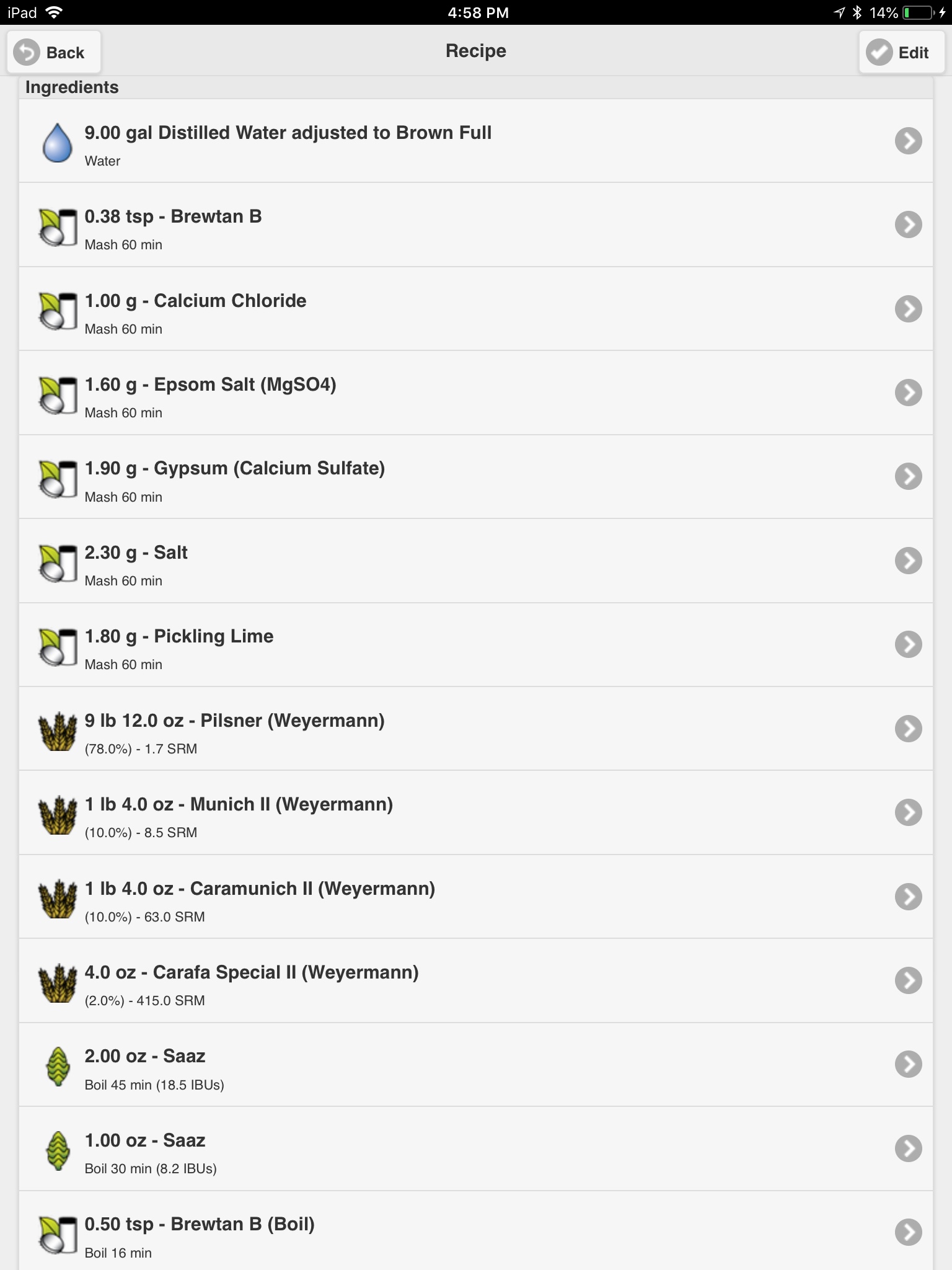
Even with its very latest version 1.22 release, BW still has nagging issues with regard to radically changing acid addition forecasting with respect to alterations made to the mashes "water to grist ratio". I just looked at straight DI (OK, let's accept it as good quality distilled) water mashes, while varying the water to grist ratio, and I got the following output for the case of mashing a simple grist consisting of only 10 lbs. of 2-Row Brewers, with color set to 1.8L, and with zero minerals added, wherein I adjusted the acid for each individual case so it would mash at a pH of exactly 5.4, and here is the output that BW delivered to me.
3 gal. DI Mash Water = 1.8 mL 88% Lactic Acid
5 gal. DI Mash Water = 2.4 mL 88% Lactic Acid
8.5 gal. DI Mash Water = 1.4 mL 88% Lactic Acid
3 gal. DI Mash Water = 3.5 oz. Acid Malt
5 gal. DI Mash Water = 4.7 Oz. Acid Malt
8.5 gal. DI Mash Water = 3.0 Oz. Acid Malt
Since DI or distilled water has effectively nigh on zero buffering capacity the results (the acid quantity required to hit a mash pH of 5.4) achieved for one water to grist ratio should quite closely match those of any other reasonable water to grist ratio. So if (for example) 2.4 mL of lactic acid is correct, then 1.4 mL can't possibly also be correct, and if (for example) 4.7 Oz. of Acid Malt is correct, then 3.0 Oz. of acid malt can't possibly also be correct. These are huge deviations, and in the real world they must noticeably impact the final mash pH.















































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)