I made a 4.5 gallon partial-mash batch of bohemian pilsner today. BeerSmith said it should have had an estimated OG of 1.044
Three days ago I made a gallon starter of wyeast bohemian pilsner yeast.
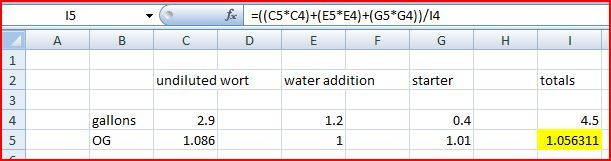
My original wort (including sparge) was about 3 gallons. I then added the gallon starter, and then topped off the last 0.5 gallons with filtered water to the fermenter.
I forgot to take an OG before adding the starter and topping off. I just took. a specific gravity reading and I'm at 1.024 not the predicted 1.044.
This is already not a very strong recipe, with a predicted ABV of 4.3. I'm worried that I had very poor conversion, and am considering boiling a concentrated bit of DME to add to the wort. For my mash I just held the grains at about 156 for 30 minutes in 2 gallons, and then sparged at 170 for 40 min in 1 gallon, and then combined the two pots, brought to a boil and added the LME then hops.
Or maybe I'm just over reacting? And a gallon starter is likely to have lowered my (perfectly converted) wort from the 1.044 it really was (and would have been had I measured at the correct time) to the now just fine 1.024?
Help?!
Three days ago I made a gallon starter of wyeast bohemian pilsner yeast.
My original wort (including sparge) was about 3 gallons. I then added the gallon starter, and then topped off the last 0.5 gallons with filtered water to the fermenter.
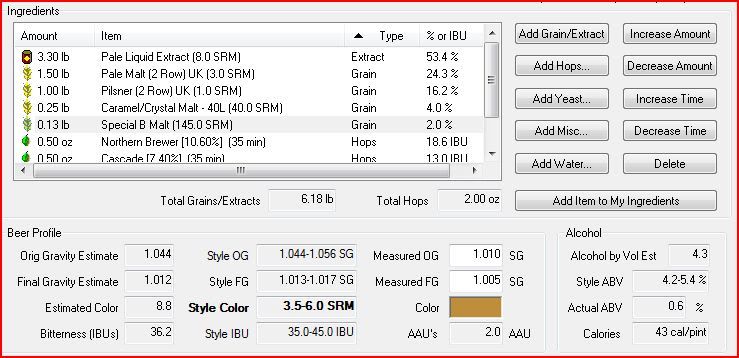
I forgot to take an OG before adding the starter and topping off. I just took. a specific gravity reading and I'm at 1.024 not the predicted 1.044.
This is already not a very strong recipe, with a predicted ABV of 4.3. I'm worried that I had very poor conversion, and am considering boiling a concentrated bit of DME to add to the wort. For my mash I just held the grains at about 156 for 30 minutes in 2 gallons, and then sparged at 170 for 40 min in 1 gallon, and then combined the two pots, brought to a boil and added the LME then hops.
Or maybe I'm just over reacting? And a gallon starter is likely to have lowered my (perfectly converted) wort from the 1.044 it really was (and would have been had I measured at the correct time) to the now just fine 1.024?
Help?!