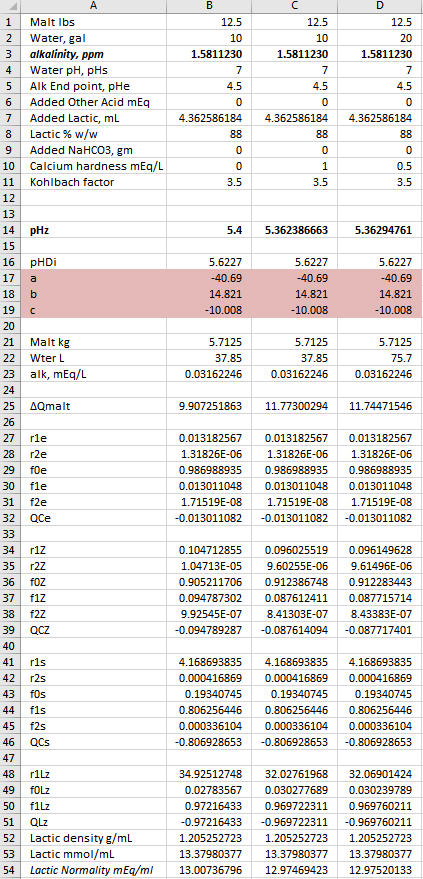
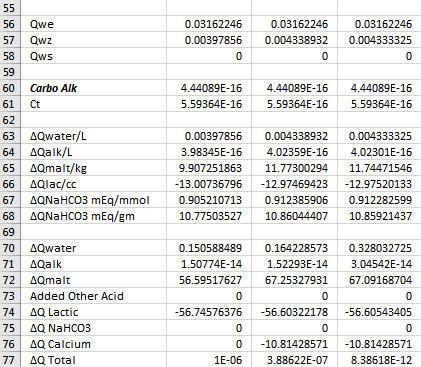
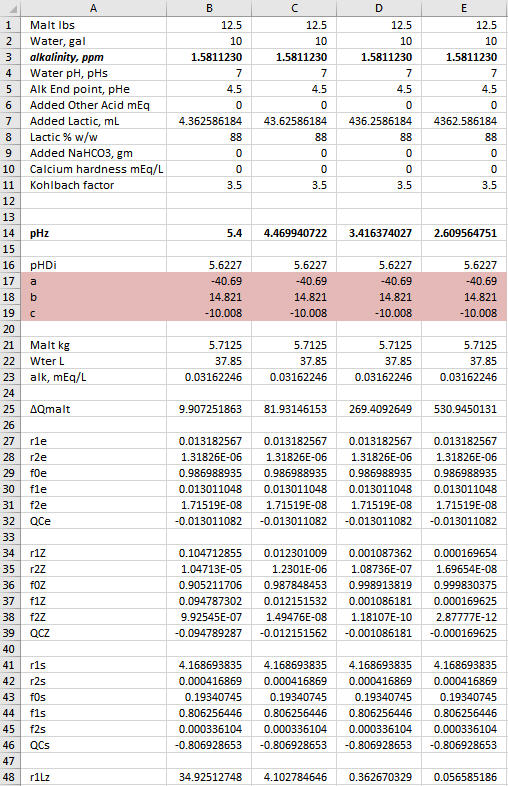
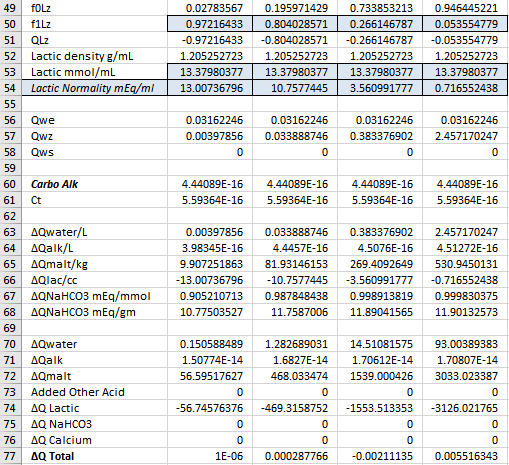
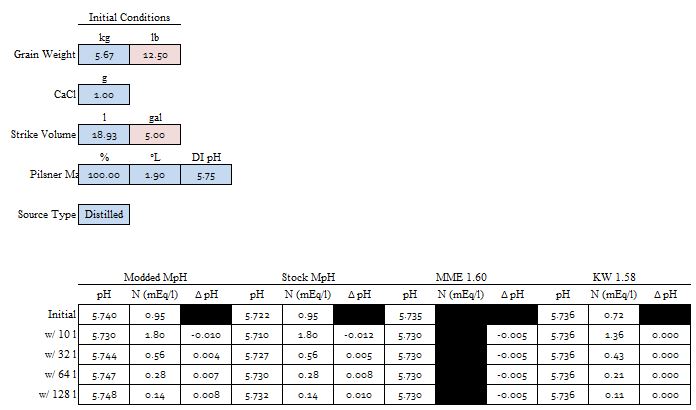
Yes, the goal is always to find the value of the sought after quantity which drives the sum of the ∆Q's as close to 0 as you can get it because in the real world the sum of the ∆Q's is 0. Now notice that ∑∆Q is larger in Col D than in the other two columns. But it is small enough. Solver is iterative. It will stop if it gets close enough to target any time but it will also stop after a certain number of iterations. If you arrive at a case with ∑∆Q larger than you are happy with just run it again. It then does another set of iterations starting where it left off last time and depending on the nature of the problem will usually find a solution resulting in a smaller residual.A.J., in row 77 both columns B and C result in very small 'Q Total' numbers, as is the goal, correct? And in both cases you ended up acidifying with the exact same quantity of Lactic Acid.
The lactic acid is exactly the same in all three cases. The goal was to show where the pH falls with 12.5 lbs malt, 0 or 37.85 mEq Ca++, 4.362586103 mL lactic acid and 10 or 20 gal DI water.
YesAs this is the case, then for the small differences in Total Q seen in row 77 for columns B and C, in reality once again we have a situation where the DI water to grist ratio is not changing the pH shifting effect by very much at all, only this time with regard to the fixed quantity of added calcium mineralization and the variable DI mash water quantity. Do you see this essentially as I do here?
Are you noticing a trend here? Kai knows what he is doing. Too bad he doesn't contribute here any more. He used to.If so, then (just as I have done for my pending Mash Made Easy version 1.60) most others will need to alter their mash pH predicting software accordingly, but the Kaiser Water Calculator is vindicated.








































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)