Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I'm asking for A.J. DeLange to provide his input here.
First Mash Scenario:
---------------------------
BIAB
No Sparge
No added mineralization
10 gal. DI Water
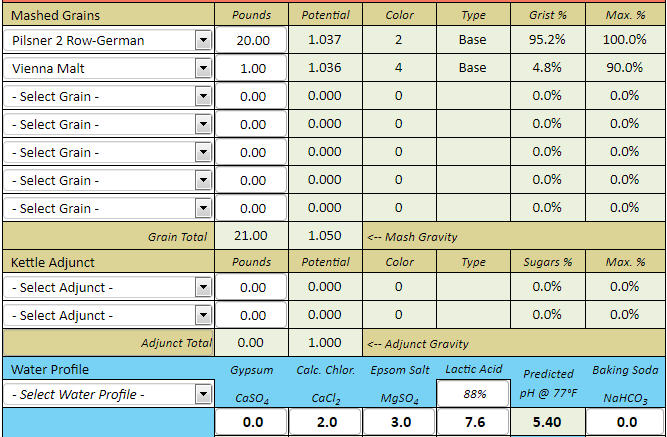
12.5 Lbs. of 1.7L European Pilsner malt
Second Mash Scenario:
--------------------------------
10 gal. DI Strike Water
10 Gal. DI Sparge Water
No added mineralization
25 Lbs. of 1.7L European Pilsner malt
Given the above, the question for A.J. DeLange is this:
If it is found that for scenario 1 above the adjustment required to mash at a nominal target pH of 5.4 is roughly 6 mL of 88% lactic acid, then can it be assumed in advance from this that for scenario 2 above the lactic acid adjustment required to hit the same 5.4 mash pH should be roughly 12 mL of 88% lactic acid?
Reason for asking: I've noticed that for the Brewers Friend online mash pH calculator and my Mash Made Easy spreadsheet the above relationship with regard to the requisite acid quantity addition doubling with grist weight doubling in order to hit the same mash pH target holds to be true, but for some other of such software it does not hold true, and for yet other of such software it does not hold to be even close to remotely true. I'm asking you to resolve this dilemma, as it seems to me to be a rather serious one.
First Mash Scenario:
---------------------------
BIAB
No Sparge
No added mineralization
10 gal. DI Water
12.5 Lbs. of 1.7L European Pilsner malt
Second Mash Scenario:
--------------------------------
10 gal. DI Strike Water
10 Gal. DI Sparge Water
No added mineralization
25 Lbs. of 1.7L European Pilsner malt
Given the above, the question for A.J. DeLange is this:
If it is found that for scenario 1 above the adjustment required to mash at a nominal target pH of 5.4 is roughly 6 mL of 88% lactic acid, then can it be assumed in advance from this that for scenario 2 above the lactic acid adjustment required to hit the same 5.4 mash pH should be roughly 12 mL of 88% lactic acid?
Reason for asking: I've noticed that for the Brewers Friend online mash pH calculator and my Mash Made Easy spreadsheet the above relationship with regard to the requisite acid quantity addition doubling with grist weight doubling in order to hit the same mash pH target holds to be true, but for some other of such software it does not hold true, and for yet other of such software it does not hold to be even close to remotely true. I'm asking you to resolve this dilemma, as it seems to me to be a rather serious one.
Last edited: