I've read through a few books & bru'n waters tutorials on water chemistry and have been tweaking my H20 with the help of Beersmith. I've been quite happy so far with how things have turned out, but my salt additions to date have been relatively small. Next recipe on my list is this big imperial stout.
Local water:
Ca=45 Na=6 Alkalinity=125
Mg=2 S04=60
Cl=4 Hc03=152
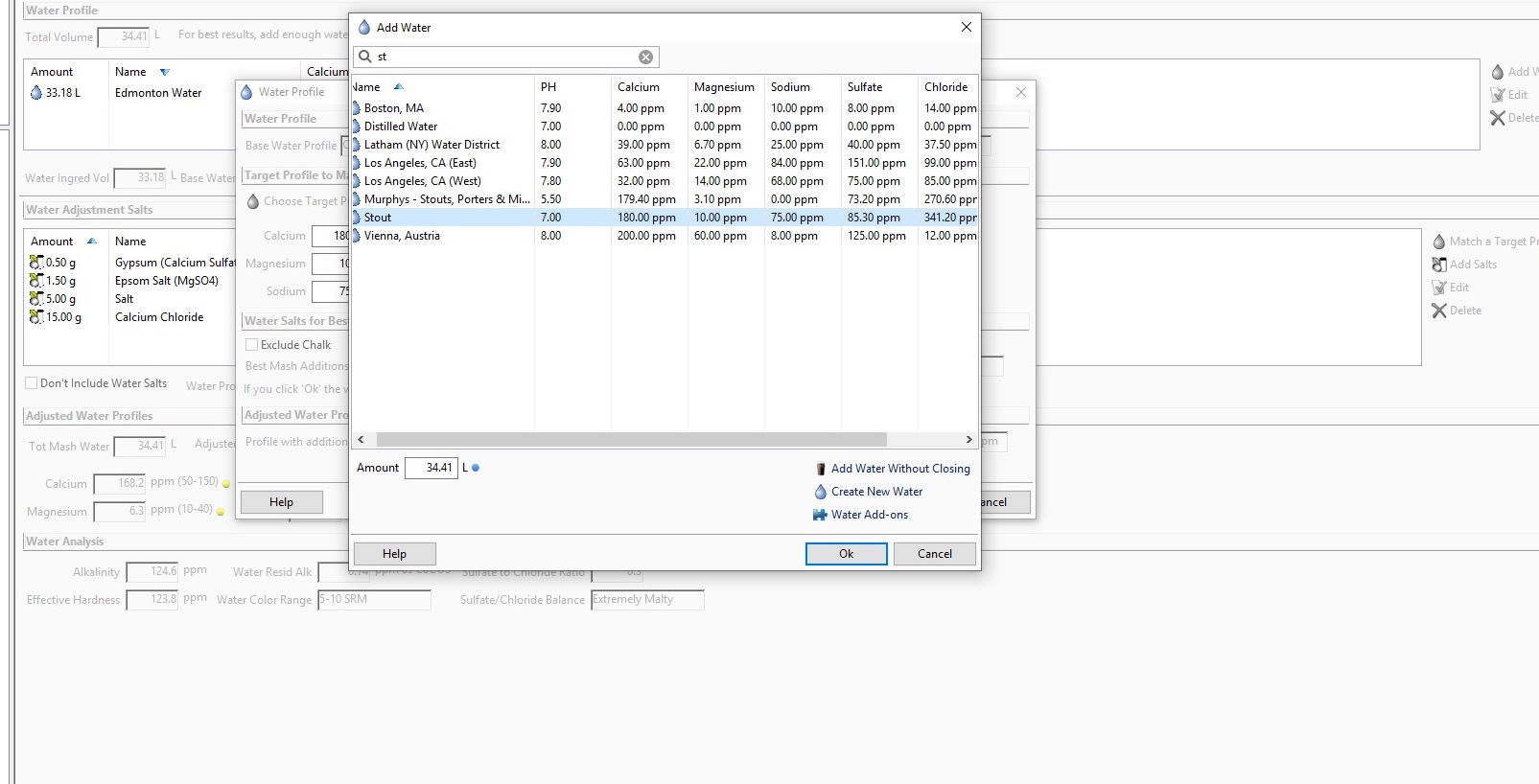
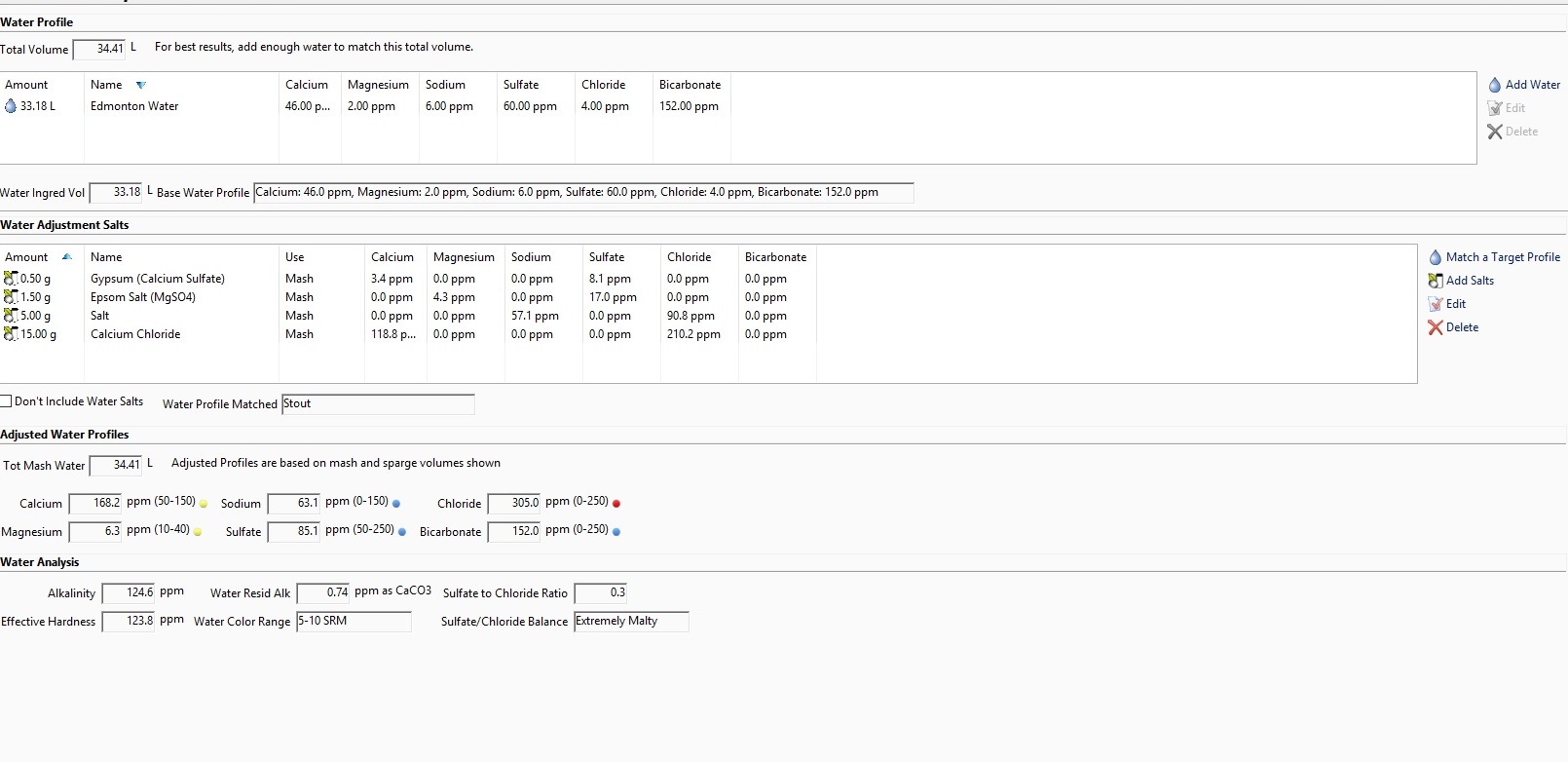
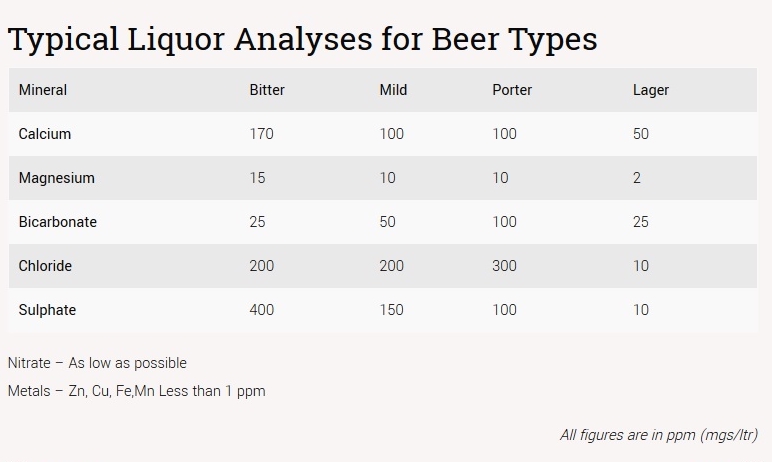
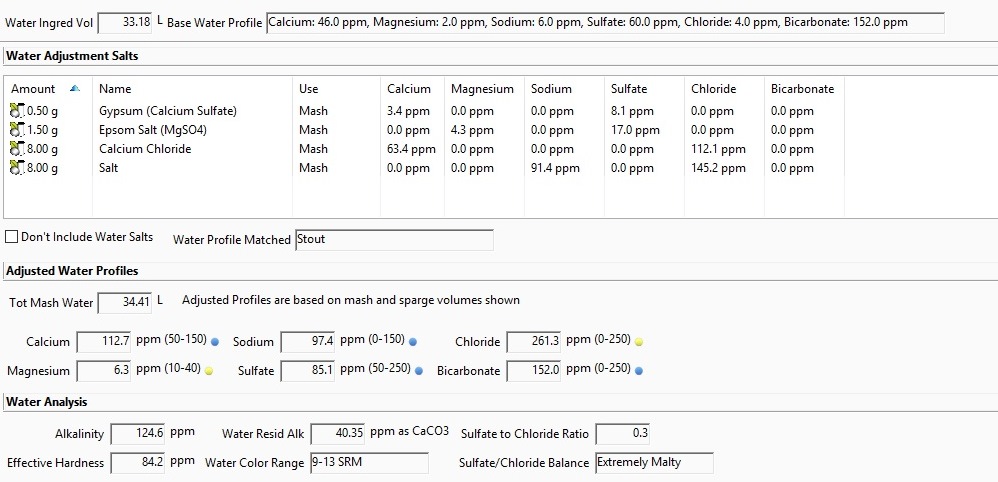
Using Beersmith to target the "Stout" profile has me adding ~15g of CaCl mainly to hit the profile's Cl level of 340.
I BIAB and have been typically adding any salts to my mash (water vol for this batch is 34L/9gal) because they dissolve more easily. No past issues with efficiency, but i've read CaCl can cause problems with conversion in the mash at higher levels.
Will this 15g if added during the mash likely cause me issues? Should I be considering adding some at another point in the brew? Targeting a different profile maybe?
I'm still a bit of a novice at tweaking my water, any advice from people smarter than me tweaking their BIAB water would be appreciated.
Local water:
Ca=45 Na=6 Alkalinity=125
Mg=2 S04=60
Cl=4 Hc03=152
Using Beersmith to target the "Stout" profile has me adding ~15g of CaCl mainly to hit the profile's Cl level of 340.
I BIAB and have been typically adding any salts to my mash (water vol for this batch is 34L/9gal) because they dissolve more easily. No past issues with efficiency, but i've read CaCl can cause problems with conversion in the mash at higher levels.
Will this 15g if added during the mash likely cause me issues? Should I be considering adding some at another point in the brew? Targeting a different profile maybe?
I'm still a bit of a novice at tweaking my water, any advice from people smarter than me tweaking their BIAB water would be appreciated.