SourLover
Well-Known Member
I have 5 gallons of golden sour that's been sitting for about 17 months. My plan is to transfer 3 gallons onto tart cherries, and then bottle the balance of the base beer.
The calculator that I use says the remaining CO2 in the beer is 0.83 volumes, but after 17 months that doesn't seem like a good number. Does anyone have an guess as to how much might be remaining, or perhaps someone has a formula/method that they use? I'd really like to get the carbonation right on the base beer as it has turned out very well.
For the 3 gallons that is going to referment I'm assuming that the 0.83 volumes is a good number to use.
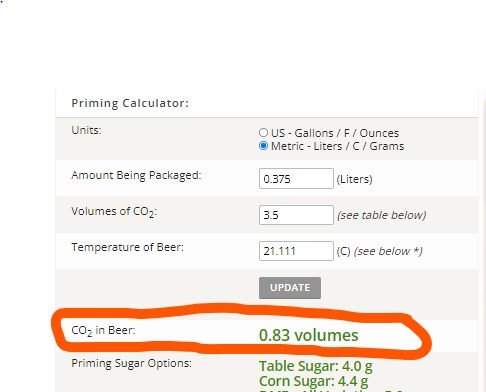
The calculator that I use says the remaining CO2 in the beer is 0.83 volumes, but after 17 months that doesn't seem like a good number. Does anyone have an guess as to how much might be remaining, or perhaps someone has a formula/method that they use? I'd really like to get the carbonation right on the base beer as it has turned out very well.
For the 3 gallons that is going to referment I'm assuming that the 0.83 volumes is a good number to use.



