Science is not my strong suit. Please don't laugh at me. I'm married to a scientist and I get enough of that at home.
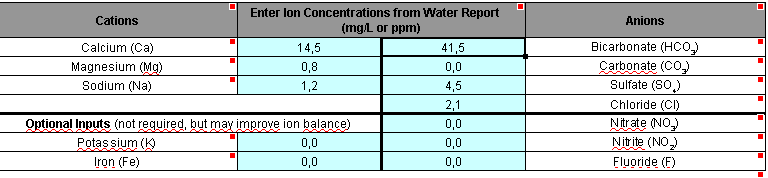
My water report lists "bicarbonate as CaCO3" but BeerSmith is asking for bicarbonate as HCO3. Can one derive the former from the latter? Does any of this really matter since apparently I should be using EZ Water calculator?
Also, is the convention to use averages when determining the constituents of one's water? I live in the Bay Area and my water report lists what sometimes seem a large range of values (but may actually be insignificant...I'm still new at this and trying to figure that out).
Thanks for the help. If any of you need any help with 19th century post-Hegelian German philosophy or the history of Continental political thought I'm your man. :cross:
My water report lists "bicarbonate as CaCO3" but BeerSmith is asking for bicarbonate as HCO3. Can one derive the former from the latter? Does any of this really matter since apparently I should be using EZ Water calculator?
Also, is the convention to use averages when determining the constituents of one's water? I live in the Bay Area and my water report lists what sometimes seem a large range of values (but may actually be insignificant...I'm still new at this and trying to figure that out).
Thanks for the help. If any of you need any help with 19th century post-Hegelian German philosophy or the history of Continental political thought I'm your man. :cross: