I've never done this one, but I've done IBUs which I think might be similar. Can you describe the method and your process?
Anatidaephobic,
The ASBC 6A process is to take 2.5 g of hops, place in a Toluene solution, spin for a maximum of 40 minutes, let the liquid and solid separate. Take a aliquot (small quantity) and dilute with Methanol. This is solution A. This dilution is then diluted again with a 6M (corrected after 10/8 post) solution of NaOH Methanol that was made prior - this dilution is called dilution B.
The same thing happens for the baseline solution except you leave out the hops and proceed the same way. I call this dilution C.
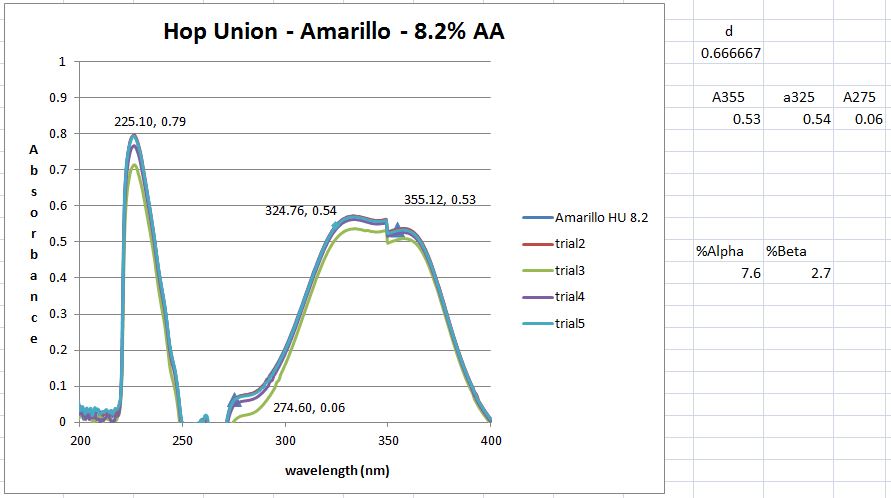
Then dilution B and C are placed inside the quartz cuvette and spectra analysis from 400 nm wavelength down to 200 nm is carried out. The equipment is run in a double beam mode utilized solution C as the baseline and subtracts the result from B. That is the trace you see.
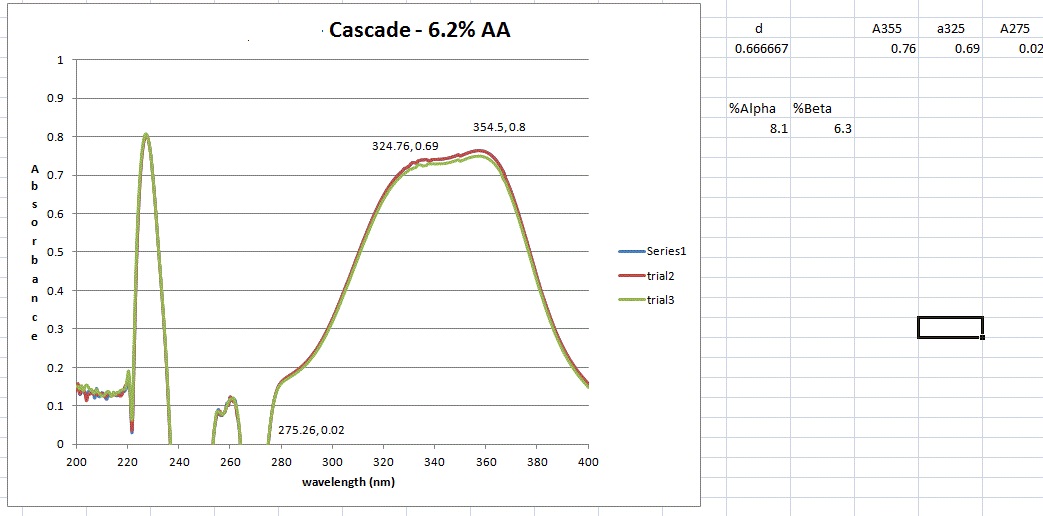
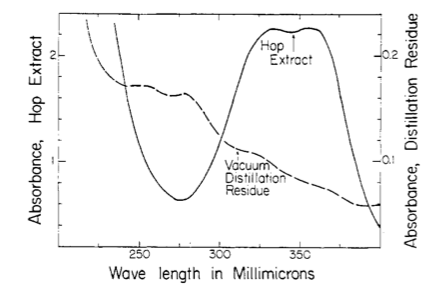
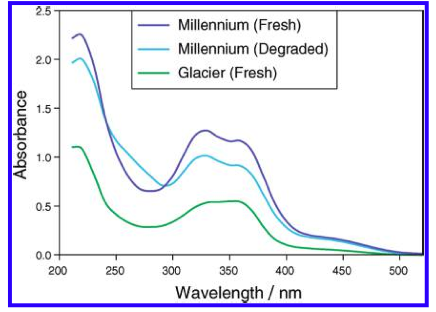
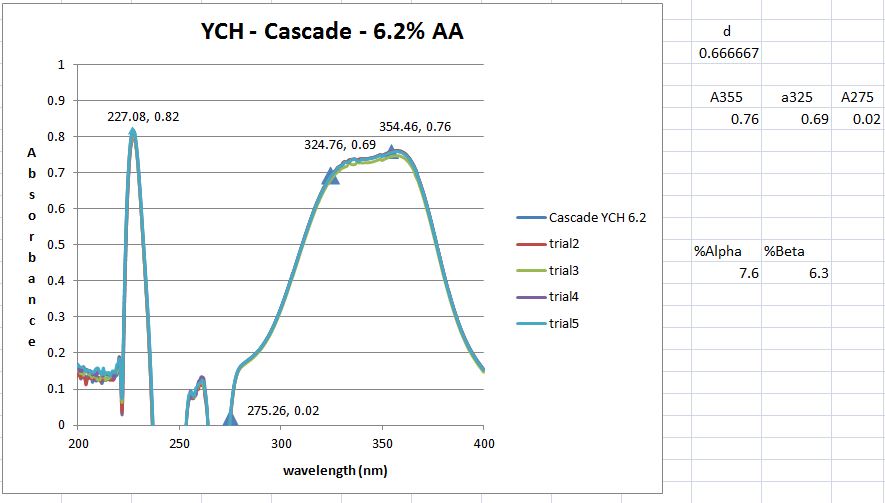
However, I don't get the peaks at 355, 325 and 275 nm that the standard says I should. But maybe I shouldn't have a peak here and just utilize the absorbance values as I did. However, when those calculation are done I get 8.2 AA which I think is to high as the value from the package is 6.5. It could be possible however ....
I guess what I was looking for was for someone to have run though this process (ASBC 6A) and send me an absorbance trace of what they get. This way I could say mine is correct or its not and learn a bit more how labs actually do this type of AA / BA analysis.
I'm thinking that a lab is doing an alpha acid analysis has some calibration standard and an output showing what the absorbances are at these (3) wavelengths. Then its just a plug and chug. Never having done this before and not having an organic chemistry background, leaves me in the dark about what might be happening as a result of this process.
Thanks for your interest. If you need further detail, PM me and I'll respond.
BB