Holter
Well-Known Member
Hey everyone. Sorry for the basic question, but I am just looking for someone to help me interpret the water quality in my city as I start to learn more about water as a brewing ingredient.
Here is the link to my report: http://www.gswater.com/csa_homepages/documents/CulverCity061611.pdf
And here are the values that I pulled from it:
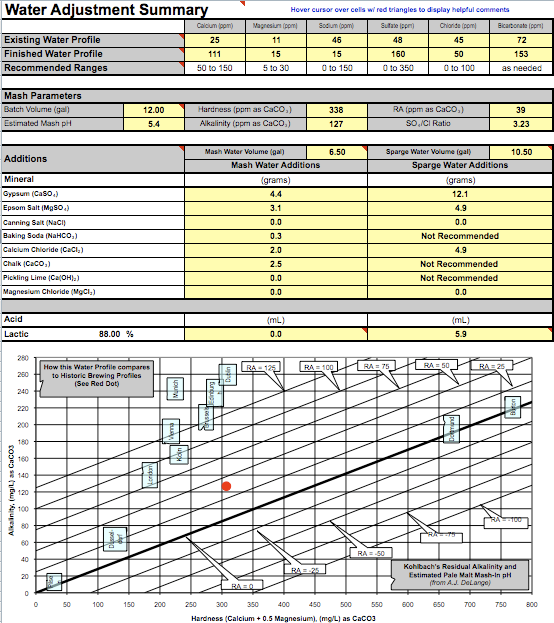
Calcium 53
Alkalinity 106
Magnesium 22
Sodium 85
Chloride 88
Sulfate 168
Ph 8.0
My first question is, did I pull the right numbers? Did I miss any? Is there anything in the report that is in any way alarming?
I typically brew pale ales, ambers, ipa's and iipa's. How would you interpret these numbers if you were brewing one of those styles? I'm trying to figure this out myself by reading this forum but the chemistry level is a bit beyond me currently, but seeing how others would modify my water will help me learn.
Thanks in advance-
Here is the link to my report: http://www.gswater.com/csa_homepages/documents/CulverCity061611.pdf
And here are the values that I pulled from it:
Calcium 53
Alkalinity 106
Magnesium 22
Sodium 85
Chloride 88
Sulfate 168
Ph 8.0
My first question is, did I pull the right numbers? Did I miss any? Is there anything in the report that is in any way alarming?
I typically brew pale ales, ambers, ipa's and iipa's. How would you interpret these numbers if you were brewing one of those styles? I'm trying to figure this out myself by reading this forum but the chemistry level is a bit beyond me currently, but seeing how others would modify my water will help me learn.
Thanks in advance-