sawbossFogg
Well-Known Member
Anyone have a good reason for naturally conditioning kegs when force carbonating is so much faster and more reliable?

It's been on my mind as I like to prime my kegs w beergas, which I of course cannot do naturally. I don't force carbonate in my keg fridge because I believe colder liquids will require more time to get gases into solution.
Jaun, I really like serving nitro beer and I'm glad you're interested, but I have a couple strong disagreements, based on experience. First, when breweries condition beer in Brite tanks, if the beer is going to be served on Beergas it MUST be conditioned that way. That is because CO2 is much more soluble than nitrogen and what you have in a bottle to push beer with is simply used for that, to push, whats in solution is a different matter. Heres an example, if you buy a keg of carbonated beer and attempt to serve it with beer gas, even at low pressure you'll be putting carbonated beer through a nitro faucet and you'll get foam! I tried this many times and until I asked several different breweries how I should go about it, I didn't get it right. The only functional method is to flatten carbonated beer and start over with beergas to get nitrogen into solution. This method takes about 4 days for a qtr barrel (at room temp). I find that my 40 cu ft bottle of beergas lasts a very long time and it only costs $30 to fill so...
As far as gas being more or less soluble at differing temps in liquids, I think thats a simple matter of physical chemistry whereby colder liquid molecules are slower and more dense. Breweries probably keep their brite tanks at fridge temps for freshness, but if you want to speed up physical processes, warm things up. Hey, I'm always up for learning something new, maybe there's call for another thread.
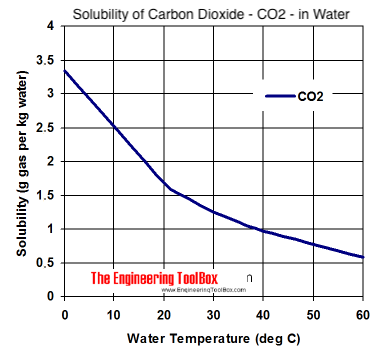
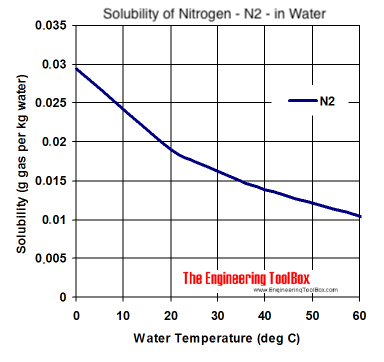
I have strong disagreements too Juan, and would also like to add that you are an *******
Ok thanks for the toolbox graphs, sounds good. You can pour your carbonated beer through your nitro faucet if you like, but I've tried it quite a bit and its ****ed, so I'll stick with 75% nitrogen beer gas because I can afford it
Ok you've got me considering carbing at a lower level and pushing it through my nitro faucet, which begs the question; Is every pub and manufacturer throughout the world that uses beer gas a complete *******?
Gasses being more soluble in liquids at colder temperatures is a well known fact, and an example of Henry's law. Even if you're not familiar with chemistry or Henry's law, it should be obvious just by looking at any carbonation chart. By warming things up, you're both slowing down the carbonation process, and reducing the total carbonation possible at a given pressure. I keep most of my beers at 40°F and 11 psi for a carbonation level of 2.4 vol. To get that same level of carbonation at 65°F I'd have to increase the pressure to 27 psi.
Ok you've got me considering carbing at a lower level and pushing it through my nitro faucet, which begs the question; Is every pub and manufacturer throughout the world that uses beer gas a complete *******?
And...?
Nonetheless, my original post, like so many others I have subsequently found and read, was asking for opinions regarding the merit of natural vs force carbonation.
Ok you've got me considering carbing at a lower level and pushing it through my nitro faucet, which begs the question; Is every pub and manufacturer throughout the world that uses beer gas a complete *******?
the main reason i naturally carbonate, though, is to save my keezer space for my carbonated kegs. I can naturally carbonate while the keezer is full and the c02 expenditure to force carbonate is greater at higher temperatures.
Well, there's good learning here for me thanks. Of course Nitrogen gas can dissolve into solution as logic and Jaun's graph from the engineering toolbox would suggest.
You'll find that N2 is considered insoluble (hard to dissolve) into beer and is thus used for high pressure draft systems for overcoming resistance in long haul lines because it won't affect carb volume like using standard CO2 in these situations.
Even the graph covers that. N2 doesn't carbonate beer at any of the pressures used to dispense it.
The N2 graph references solubility as a function of temperature. Please, Sparky enlighten me.
N2 doesn't carbonate beer at any of the pressures used to dispense it.
At about a hundredth that of CO2. Perhaps you missed
Reading is fundamental.


In fact for interest sake, i just poured a bit of an Imperial Stout that sat at 45 F for 4 days at 30 PSI and yeah its pretty much flat, whereas if it were CO2 it'd be overcarbed. The last chart/intel I need to find is what are ideal carb units for preparing to push w beer gas.
Ahh, got it. Not saying your wrong, just working through it. In fact for interest sake, I just poured a bit of an Imperial Stout that sat on the beer gas at 45 F for 4 days at 30 PSI and yeah its pretty much flat, whereas if it were CO2 it'd be overcarbed. The last chart/intel I need to find is what are ideal carb units for preparing to push w beer gas are, in the past I've had over-carb issues.
And since were being ********, I'll mention that it's also fundamental not to crap the bed in the World Series!
At about a hundredth that of CO2. Perhaps you missed
Reading is fundamental.
The whole reason nitrogen is used at all is BECAUSE it barely dissolves (it will a tiny bit, but it's negligible). It allows you to push beer at the higher pressures required due to the restrictor plate, WITHOUT the subsequent overcarbing that you'd get if you used solely CO2 at those pressures.
It's not because the nitrogen itself gives the beer a different quality - ANY insoluble gas (like argon, as previously mentioned) will accomplish the exact same thing. In fact, 100% argon (and less frequently, 100% nitrogen) is used to push wine and other still beverages through a draught system for precisely the same reason... since it doesn't dissolve, it won't start carbonating (or otherwise make sparkling) the wine no matter how long you keep it on the gas for.
to answer the original question......
after reading an interesting thread on pressure fermenting....i decided to try my hand at it.
i had experience keg conditioning, which to me is dosing the already fermented beer that's in the keg with corn sugar, beet sugar I.E. whatever a person might use for priming.
force carbonation, shoot the keg with CO2 at specified pressure kept at controlled temp for a period OR roll the keg around let it set and check by tapping.
have done both of these methods but after reading the aforementioned article i decided to ferment till bubbler was showing only one bubble @ minute. then rack over to keg add 1/4 C priming sugar...i use beet sugar.
this kicks the pressures up to just a hair below 40 psig on my spunge valve.
pressures may have gone to 40 psi and activate the preset blow-off but if so i never saw the guage at 40. so far i haven't been around when the blow off has occured, if ever. so i'd say mostly 37-39ish...psig in keg.
i usually leave the beer under pressure for several weeks....then cold crash for a couple days...release the pressure and tap.
i'm VERY happy with this method. the cascading small bubbles and great head to almost the empty class is great. it is especially enjoyable in a dark to medium colored beer. one would swear it was beer gas carbed poured thru a creamer tap.
also this has helped to cut down on gas useage.
since my experience with this method i don't carb anyother way.
yes there is a small amount of trub in the first glass but if i'm careful and not to concerned about every last drop from the carboy the amount will be small and worth the effort and the beer, from then on is Kystral klear. uless a wit or hefe.
GD
And if I were to be at a predicted ~2 Volumes CO2 the worksheet you've shared suggests dispensing at 17 PSI.
Which part doesn't sound right?
Lastly, reading other threads sounds like for force carb it gets a bit artsy in terms of applying a given psi at a given pressure for x amount of time in some shaking or lap roll method. The temp and pressure charts are great, but for how long and with what method still seems a matter of debate.
Juan, yeah at 25% CO2, 38F, 2Vols, 7kft, 8%ABV I'm looking at 75 PSI, so yeah thats ridiculous, huh. If I bring it down to 1 Vol that puts it at 30 PSI which acceptable, hmm!
Last dumb question; I like to set it at a pressure and wait, question: does it need to stay at that pressure on the regulated tank or like I have my stout now, pressure applied, removed and stowed? Seems like it'd need to be hooked up to work toward equilibrium over time, but I don't have multiple CO2 regulators. Possibly periodic exposure and a little rolling is in order?
Enter your email address to join: