hophashallday
New Member
- Joined
- Aug 1, 2021
- Messages
- 3
- Reaction score
- 0
Hello, I recently moved to a new part of the country. The local water authority is really helpful with data on the water composition, but I'm having a little trouble making sense of it.
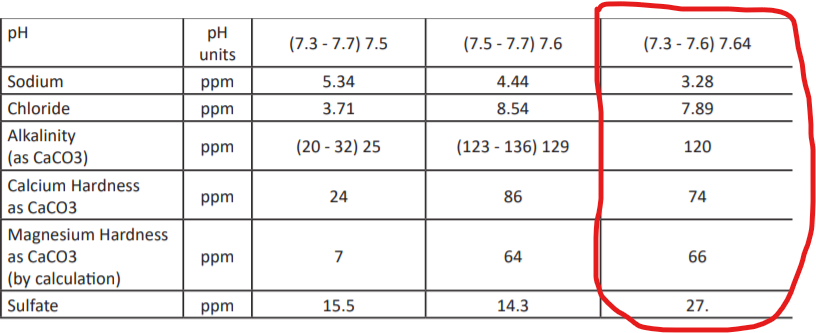
Here is the report with my the column from the source that serves my home circled:
According to calculations from Palmer's BYO, this is how it shakes out in terms of concentrations of important minerals:
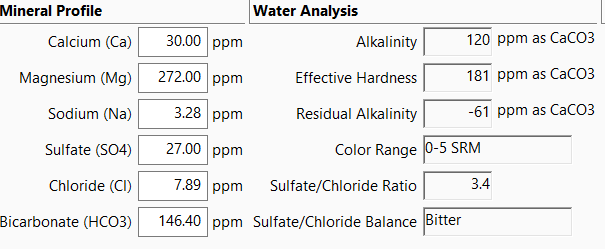
Is this accurate? That is an insane amount of mg and, if accurate, not at all suitable for brewing, right? FWIW, my home tap water does appear to be ridiculously hard in everyday life. I'm thinking I will need to just go the RO route. Truth be told, I've never much messed around with tap water since I did not have access to good data about its composition. So, this is the first time I've actually tried to figure it out. Thanks in advance for any help or correction.
Here is the report with my the column from the source that serves my home circled:

According to calculations from Palmer's BYO, this is how it shakes out in terms of concentrations of important minerals:

Is this accurate? That is an insane amount of mg and, if accurate, not at all suitable for brewing, right? FWIW, my home tap water does appear to be ridiculously hard in everyday life. I'm thinking I will need to just go the RO route. Truth be told, I've never much messed around with tap water since I did not have access to good data about its composition. So, this is the first time I've actually tried to figure it out. Thanks in advance for any help or correction.








































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)






