arturo7
Well-Known Member
Fear not g-510, we shall find a way!

is there any way to get Mg without overloading SO4?
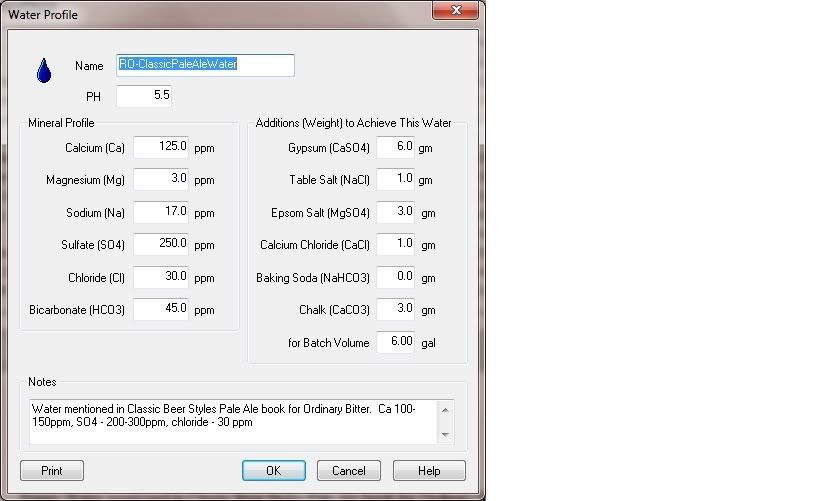
to emulate, say, milwaukee water (47ppm Mg, 26ppm SO4) you need relatively high Mg and relatively low SO4.
Is epsom salt the only way to get Mg? It has too high levels SO4....















I imagine those that know WTF goes on with all of this chemistry are sitting on the sidelines getting a good chuckle out of this thread.
When adjusting your water you are really trying to do these 3 things at once:
#1. Get the Residual Alkalinity (RA) of your water to a level that coincides with the color (SRM) of your recipe. This is to ensure that your mash will be at the proper pH.
#2. Get the Cloride to Sulfate ratio within a range that corresponds to the style you are brewing (ie. bitter, balanced, malty). This is will enhance flavor.
#3. Get the individual minerals (Ca, Mg, Na, Cl, SO4) to acceptable (or even desirable) levels..
It's like looking for a recipe generator that looks for an style guideline input and spits out a recipe. Boring.
What you're looking for is Brewater3.0. Personally, I like having to manually tweak additions because it teaches you the effects of each addition real time.
It's like looking for a recipe generator that looks for an style guideline input and spits out a recipe. Boring.
What I'm looking for is something that will give me the salt additions for a given beer style. How is that boring?
This calculator uses an algorithm to find the optimal additions. Unfortunately, it has no provision for RO water or adding a new water profile.
http://lyra.cs.virginia.edu:8080/~asb/beer/water.html

What I'm looking for is something that will give me the salt additions for a given beer style. How is that boring?
This calculator uses an algorithm to find the optimal additions. Unfortunately, it has no provision for RO water or adding a new water profile.
http://lyra.cs.virginia.edu:8080/~asb/beer/water.html
I'm all for having a reference table. I'm just making a case for doing it the hard way for a few batches so you can get a better understanding for what each salt does. It's the same argument I make for learning how to figure out efficiency on paper before relying on software for the rest of your brewing career.
Thats a reasonably safe assumption. RO strips about 95% of everything, so if your water is reasonable, it should be pretty similar across the board (sorry phoenix and your 1500ppm tds)