I borrowed that wording from Carroll. In fact in terms of percentage change in Henry coefficient with temperature nitrogen is more sensitive but because nitrogen is so much less soluble than CO2 the change in level of dissolved N2 changes much less which is clearly what he meant as he has a graph illustrating this. Between 0 and 15 °C the dissolved level of CO2 under 1 bar partial pressure changes by 1.307 grams. The level of N2, conversely, under 1 bar partial pressure of nitrogen, changes by only 5.7 mg between 0 and 15 °C.actually both CO2 and N2 solubility depends on temperature similarly - with a drop of 2.5-3 from 0C to 40C. Overall CO2 is about 100 times more soluble than N2 (3g/L for CO2 and 0.03 g/L for N2).
This means that bubbles in your beer are almost entirely CO2 (99% or maybe 97% - depending on gas composition and level of saturation reached).
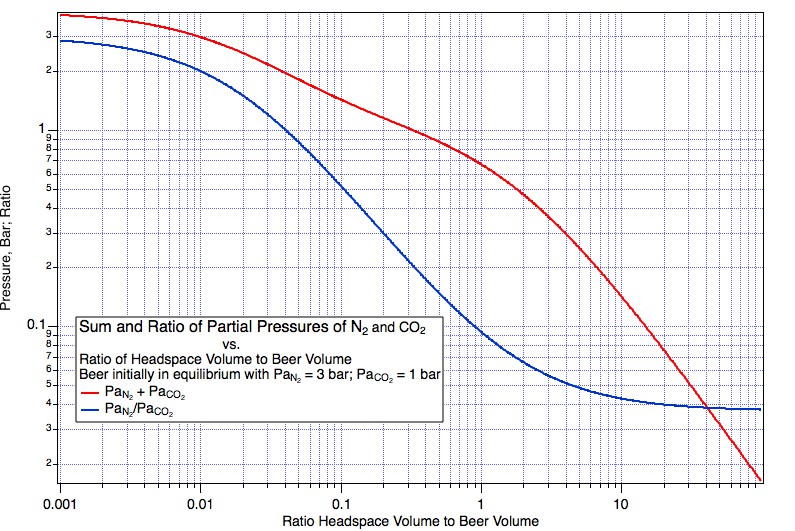
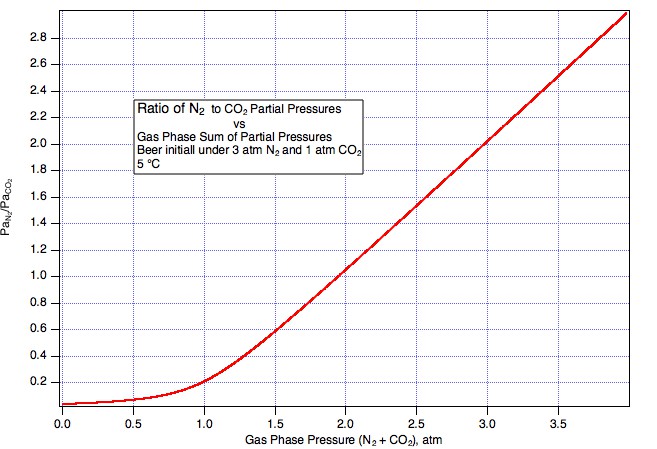
So we take some ungassed beer and put it under 3 atm (to make the math easy) beer mix with PaCO2 = 1 atm (abs) and PaN2 = 2 atm (Guiness is served with a 25% mix). Things come to equilibrium at, lets say, 5° C and we have 1*44000*CO2KHy(5) = 3041.54 mg/L dissolved CO2 and 2*28000*N2KHY(5) = 47.8879 mg/L N2. Now we very carefully (reversibly, if you speak the language of thermodynamics), without disturbing it in any way draw some of the equilibrated beer into a syringe. Next we seal the inlet to the syringe and allow the plunger to come back a bit but a very small bit. What happens here? Gas, a very small amount, leaves the beer to push the plunger back thus creating a very small head space. What is the pressure of the gas in the head space? Since it is in equilibrium with beer that was in equilibrium with 3 bar in the keg, the head space pressure will be close to 3 bar in the syringe. And what will be the partial pressure of N2 in that head space? One percent of 3 bar? No, clearly it will be 66% of 3 bar i.e. the original 2 bar. Were it less than this the nitrogen would have had to stay preferentially in the beer but that, as we haven't allowed enough time for the temperature to change much, would require the Henry coefficient to have changed by some other mechanism.
I'd doubt it too but as the nitrogen content in the bubbles is 66% (in our example) and 75% in an actual Guiness serving set up the picture is far different. But, you may say, the mg of CO2 in a bubble of a given size aren't very different between pure CO2 and CO2 diluted 2:1 or 3:1 with nitrogen and that's true but you must remember that the ability of the CO2 to form carbonic acid (which is responsible for the bite) depends on the fugacity of the gas which is a function of its partial pressure. The partial pressure is reduced to 25% of what it is with pure CO2 and the fugacity will be down to something close to 25%. Less bite.I doubt that 1% Nitrogen content inside the bubbles contributes to significant reduction of carbonic bite.
I thought that too for a long time but if you taste beer drawn on nitrogen carefully I think you will change your mind. I did.I believe Nitrogen contribution in bubbles is irrelevant to the taste -
I have done this (out of N2 and who wants to run all the way out to Roberts just for a glass of stout) and have suggested people do this for years claiming you will get 95% of the effect. I'm not backing away from that position but I think I might revise the 95% number downward.you just need to create small bubbles by applying high pressure and forcing lightly carbed beer through an aperture.
The fact of the bubbles being smaller does have an effect as part of the prick is mechanical (with the rest being carbonic acid).The "smooth" taste is simply result of those small bubbles.
You can push lightly carbed beer with other gases, or mechanical devices and get the precisely same effect.
Guiness originally got into this because they wanted to be able to draw draught beer with relatively uniform head properties from the beginning of the cask to the end and they wanted the head to resemble that from a hand pump. They started with air and then moved to nitrogen which is Carroll's words led to the 'dramatic' difference a small amount of dissolved nitrogen makes. Thus while you can get a similar effect with just a hand pump you cannot get precisely the same effect. From the physics it is clear that any gas which dilutes (lowers the partial pressure of) CO2 should work in the same way. Clearly an inert gas such as argon (but not radon) would seem to be a reasonable choice for experimentation.




































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)