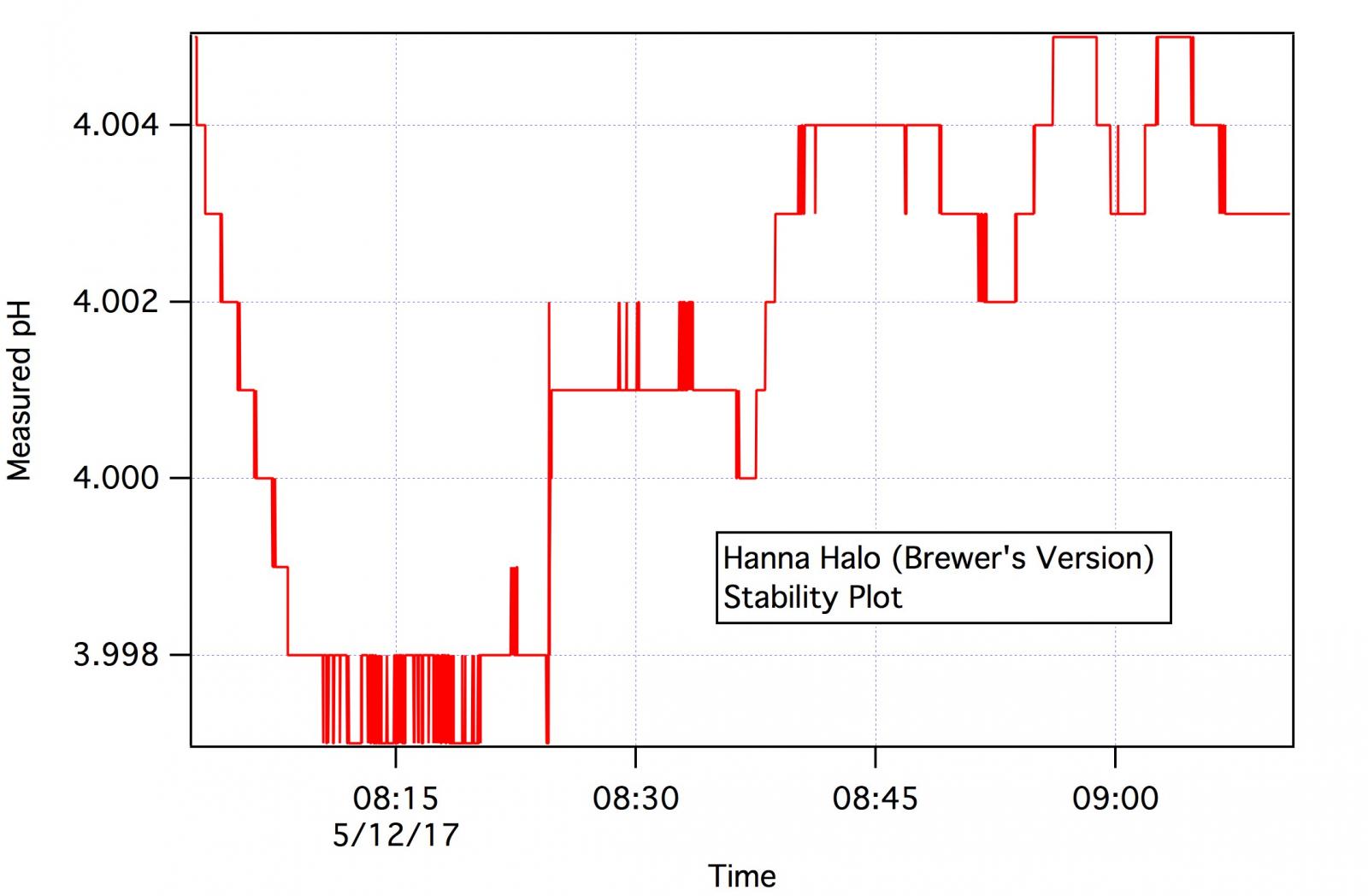
I can tell you about one problem - the one that has kept it off the list of HBT 'approved' pH meters. While the electrode is stable the calibration routine does not give the user the option to tell it when to accept a calibration reading. The meter decides when the reading is stable and accepts it whenever it's stability algorithm is satisfied. But it accepts readings too soon. If it waited another 30 -60 sec the reading would have changed appreciably. This is enough to throw the calibration parameters off.
The user should be the one to determine when to accept a calibration reading and many meters in this same price range offer that capability. This is really too bad as, given that it is inherently stable (passes the stability test - see below), this meter would make a good choice for the home brewer but this problem disqualifies it. There are workarounds but they are clumsy and who wants to fiddle with clumsy workarounds?
See
https://www.homebrewtalk.com/forum/threads/ph-meter-calibration.302256/ for more or what we mean by stability and how we test it.





























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)