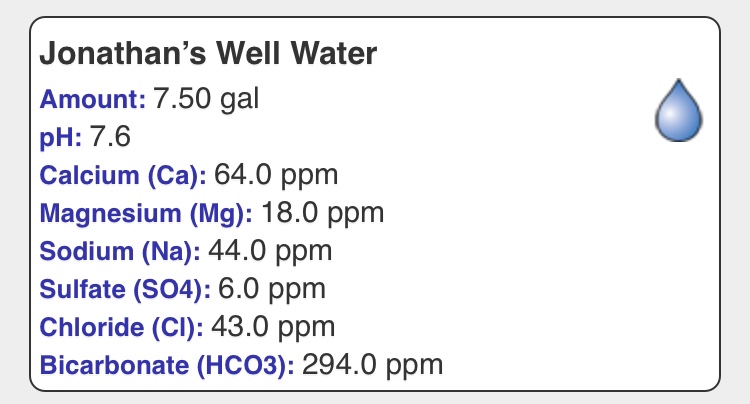
You have about 4.8 mEq/L alkalinity and would like to reduce this as much as possible. You can do that by adding 4.8 mEq/L acid which replaces each mEq of alkalinity with 1 mEq of the anion of the acid. Thus, were you to use sulfuric acid your sulfate would increase by 4.8 mEq/L (230 mg/l) and similarly for other acids. Were you to use hydrochloric acid your chloride would go up by 165 mg/L. In the UK they sell a equinormal blend of these acids which, if used would increase your chloride by 83 mg/L and your sulfate by 115 mg/L which would result in a reasonable content of those ions for many traditional British beers and is a commonly used approach to water like yours in the UK. For other beers (delicate lagers) you would not want to take this approach as your sulfate is already marginal for them and going the whole distance with chloride will get that up to where many would find it excessive. For those beers decarbonation by other means is sought. Many home brewers use lactic acid and while quite a bit of it can be tolerated before its flavor effects are noticed it does have flavor. Phosphoric acid is often used because it is pretty flavor neutral (the extra phosphate contributed by the acid is swamped by the phosphate already in the mash from the malt).
The easiest decarbonation method by far is RO but that requires an investment in RO equipment. That leaves the old traditional methods: boiling and lime precipitation. The former is easier than the latter but uses energy. Both take time. In both you first supplement the calcium in order to get it up to above the level of the alkalinity. In this well water you have 64/20 = 3.2 mEq/L Ca++ and thus need to add at least 1.6 mEq/L more. Thus even using these methods you will have increased chloride and/or sulfate and, as that isn't always wanted you are driven back towards the RO solution.
Having gotten the calcium level up the next step is to boil the water. It will turn milky and upon cooling and standing chalk will precipitate. The clear supernatant will have alkalinity (and hardness) of about 1 mEq/L which is much better than 4.8 and 1 mEq/L Ca++ (20 mg/L) isn't bad either though you may wish to add more.
In lime treatment you add lime, stir and let the chalk precipitate. You then "neutralize" the excess alkalinity (from the lime) either with acid or untreated water to pH 8.3. The result is, again, water with alkalinity of about 1 mEq/L. Lime treatment really requires a pH meter and some knowledge of what you are doing. Either method is clearly a PITA relative to drawing some RO water or dumping in some acid but in the latter case you have to accept the anion.
I'm sure this must all be confusing at this point. For that, and other, reasons I generally recommend RO. But, should you find brewing water chemistry interesting I recommend using the other methods for the learning opportunities they grant.




































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)























