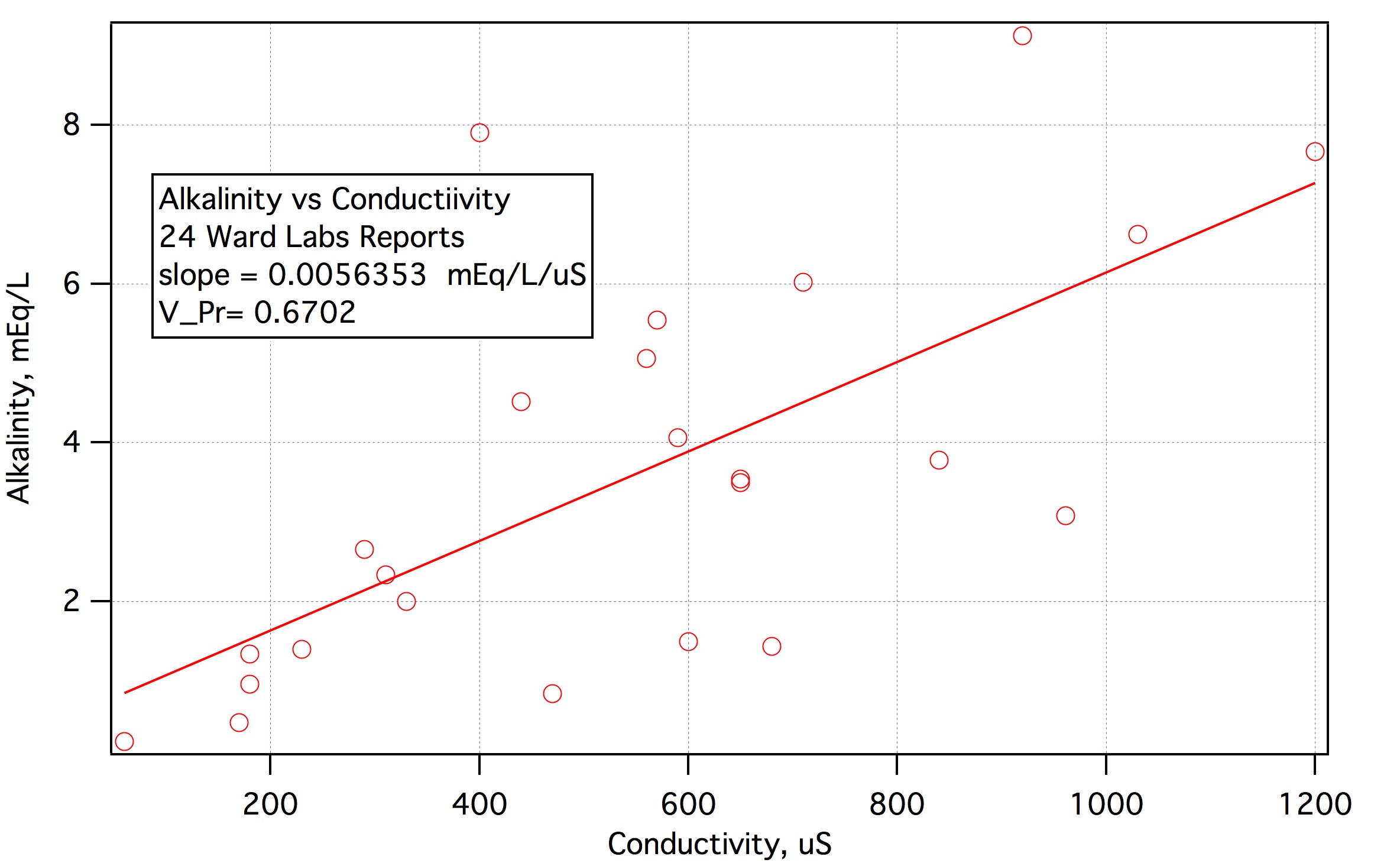
As I am sure you are aware "TDS" is estimated by measuring conductivity of the sample and comparing the conductivity to a table of conductivity vs concentration of something which is often NaCl. I also expect that you are aware that the mobility of ions, and hence the conductivity of solutions that contain them vary quite a bit between different species so that a meter whose scale reads ppm accurately for NaCl solution might not give an accurate result in a solution of Ca(HCO3)2. Recognizing this, at least one company (Myron L) has come up with a calibrating solution made up of 40% NaSO4, 40% NaHCO3 and 20% NaCl and claims that it is representative of natural waters. Thus seems a little strange to me as calcium is quite a bit more mobile than sodium and has an appreciably greater specific conductivity (12 vs 5). But we don't have much to work with here so let's suppose we put 400 mg NaSO4, 400 mg NaHCO3 and 200 mg NaCl into a liter of water. Actual TDS would then be 1000 ppm and the conductivity 1417 uS which, in a meter scaled to NaCl would read TDS 703.6. So we might suppose that if we used a NaCl based meter and it read 703.6 we could multiply that by 1000/703.6 and conclude that our sample was equivalent to 1000 ppm TDS of "442" (which is what the manufacturer calls its calibration solution based on this mix). Or we could multiply by 0.4*1000/703.6 and conclude that our sample contains the equivalent of 400/703.6 ppm NaHCO3 and finally, divide by the molecular weight of NaHCO3 to get (0.4*1000)/(703.6*84) = 0.00676791 as the multiplier to be applied to the TDS reading from a cheapie TDS meter to convert it reading to an estimate of the alkalinity (in mEq/L). Now you have to recognize that if you went out and bought a standard 1417 uS conductivity standard, and dunked your meter into it it would read 703.6 ppm which, multiplied by 0.0067691 would tell you the alkalinity of the standard is 4.76 mEq/L when in fact the alkalinity of this solution is practically 0. The use of the factor does, however, give one a path between TDS reading and an alkalinity estimate in natural waters depending on how well their alkalinity vs conductivity characteristics resemble those of 442.
Another approach would be to grab every water report that is posted on HBD and record the alkalinity and conductivity numbers. You could then fit a line through a scatterplot of alkalinity vs conductivity. You would then take a TDS reading from a NaCl based meter, convert it to us based on a table of conductivity vs NaCl concentration and the multiply the uS by the slope of the alkalinity vs. conductivity fit line.









































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)