Kai, that book I e-mailed you has a chapter on this topic.
I'll copy and paste some of it here.
--------------------------------------------------------------------------------
Changes in mash thickness (liquor/grist ratio) have significant effects on mash
performance (Hind, 1950; Hopkins and Krause, 1947; Harris and MacWilliam, 1961;
Muller, 1989; 1991; Table 4.14). Very concentrated mashes, (liquor/grist <2:1 ml/g), are
difficult to mix and pump, extract recoveries are reduced, starch conversion is slowed down, worts are more concentrated and viscous, TSN and FAN are increased and more high molecular weight nitrogenous substances remain in solution, but a lower proportion
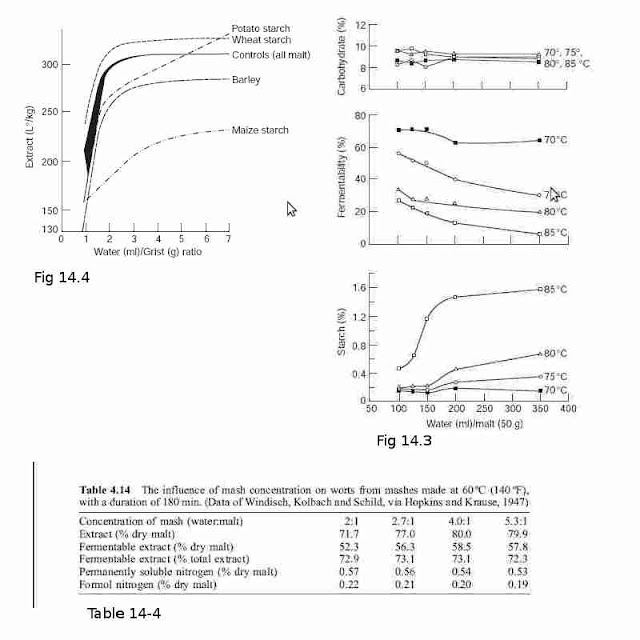
of hydrophobic peptides (relative to the amount of extract) are present, causing `high gravity' beers to have poor head retentions (Bryce et al., 1997). In the concentrated mashes both the enzymes and their substrates are more concentrated. Some enzymes (proteolytic enzymes, disaccharidases) are more stable in concentrated mashes producing higher proportions of TSN and hexose sugars respectively. At high mashing temperatures thicker mashes give worts with higher fermentabilities (Muller, 1991; Fig. 4.13). On the other hand, at `normal' mashing temperatures weaker mashes give more fermentable worts. The high concentrations of sugars and dextrins present in thick mashes can inhibit the amylases. Enzyme inhibition is due to the reduced availability of free water as well as to the sugars acting as competitive inhibitors. Brewery worts contain 0ÿ40% more soluble nitrogen than laboratory analytical worts. It was reported that mashes made with 39% solids give worts with maximum extract yields while worts with the highest fermentabilities are given by mashes made with 16ÿ32% solids. The effects of mash concentration on extract yield are also present when adjuncts are included in the mash (Harris and MacWilliam, 1961; Muller, 1991; Fig. 4.14).
As the grist hydrates water is bound, and there is a rise in temperature caused by the
release of heat (the `heat of hydration'). As the mash proceeds water is utilized in
hydrolyses, a water molecule being consumed when any bond is split. Some water is
more or less firmly bound (by hydrogen bonding) to starch, to sugars in solution, to -
glucans, to pentosans and to other substances reducing the concentration of `free' water.
In all-malt mashes and mashes made with 50 : 50 malt and barley or wheat starch the
extract recovered falls very sharply as the liquor/grist ratio is reduced below about 2.5
(Fig. 4.14). Generally, altering the liquor/grist ratio at values over 3 has comparatively
minor effects, but these are not necessarily negligible. In a particular case mashing with a
liquor/grist ratio of 2.5 : 1 gave an extract of 291 l ë/kg, while at a ratio of 7 : 1 the extract
was 311 l ë/kg. The extent of water binding becomes progressively greater as mashes
become more concentrated and there is insufficient free water to permit the gelatinization
of much of the starch. The addition of more enzymes to a very thick mash does not
quickly convert the ungelatinized starch and so does not enhance the extract obtained.
The situation with the maize starch (Fig. 4.14) is complicated because its gelatinization
temperature (70ÿ75 ëC; 158ÿ167 ëF) is above that of the mashing temperature (65 ëC;
149 ëF) and so the conversion of the starch into extract is relatively slow. The potato
starch had a wide gelatinization temperature range (56ÿ69 ëC; 132.8ÿ156.2 ëF), which
spanned the temperature of the mash, and the pattern of extract recovery was different
again (Fig. 4.14).
---------------------------------------------------------------------------------------
Then this part about the quality of the runnings as more and more is pulled.
---------------------------------------------------------------------------------------------
As run off progresses the quality and concentration of the wort declines. The last
runnings contain extract that has a comparatively poor quality (Hind, 1950; Figs 4.15,
4.16). Relative to the extract more high- and low-molecular weight nitrogenous materials,
ash (including phosphates), silicates (mostly from the silica in the malt husk),
polyphenols and astringent substances are dissolved, all these being favoured by the
increasing pH. The specific gravity of the wort rises then declines as the sparge liquor
emerges. As the wort is diluted the fermentability initially increases and finally falls
sharply. Often the pH rises, (e.g. by 0.2ÿ0.7), as the buffering substances are eluted from
the goods. The rise is particularly marked if a bicarbonate sparge liquor is used. This rise
is undesirable and should be checked and the calcium ion concentration of the liquor
should be maintained (Laing and Taylor, 1984). Experimental thick mashes (liquor/grist
2.5/1, i.e. 28.6%) would not run off unless a high concentration of calcium ions (200mg/l)
was used. Thus the last worts are weak, and are relatively rich in poorly flavoured
extractives and potential haze-forming substances. These last runnings, like the press
liquor from the spent grains (Chapter 3), may be stored hot for a short period (to prevent
spoilage by micro-organisms) and then be added to a subsequent mash to recover the
extract. However, to maintain the quality of the beer the weak wort may need to be
clarified by centrifugation to remove suspended solids (particularly lipids) and/or may be
treated with active charcoal (doses of 10ÿ50 g/hl have been suggested) to reduce the
levels of tannins, nitrogenous substances, colour and harsh flavours before it is added to a later mash (Morraye, 1938; Prechtl, 1967).
-----------------------------------------------------------------------------------
And here's some of the figure's they are referencing.
























































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)