mdboulier
Active Member
Any thoughts on this? Similar to SOFC in that it uses ice for cooling. I'm working on this for an engineering school project. This is our crude, stripped down prototype so far. (forgive the poor insulation)
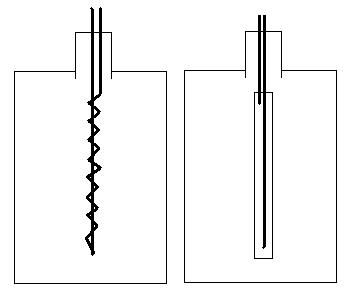
Pumps ice-cooled water through the coiled aluminum heat exchanger.
Extended thermistor on digital thermostat regulates temp
12VDC brushless water pump is compatible with the thermostat (no additional relays necessary). Depending on your choice of digital thermostat, you may be able to select temps as low as 45...or build your own control circuit....
I'm working on this with a few other engineering students. We plan to build this into a single unit with the ice water reservoir on top of the fermentation chamber and make our own control rather than use a thermostat. Thermostat should work fine for most folks though.
My buddy did a trial today, 100F to 50F in 2 hours. For perspective, a carboy submerged in an ice bath took 1.75 hours over the same temp range.
Any suggestions/comments/questions?




Pumps ice-cooled water through the coiled aluminum heat exchanger.
Extended thermistor on digital thermostat regulates temp
12VDC brushless water pump is compatible with the thermostat (no additional relays necessary). Depending on your choice of digital thermostat, you may be able to select temps as low as 45...or build your own control circuit....
I'm working on this with a few other engineering students. We plan to build this into a single unit with the ice water reservoir on top of the fermentation chamber and make our own control rather than use a thermostat. Thermostat should work fine for most folks though.
My buddy did a trial today, 100F to 50F in 2 hours. For perspective, a carboy submerged in an ice bath took 1.75 hours over the same temp range.
Any suggestions/comments/questions?