I'm on my third batch of BOMM in half as many months.
See this thread for the original recipe and many great notes on making a wonderful mead.
https://www.homebrewtalk.com/forum/threads/brays-one-month-mead.429241/
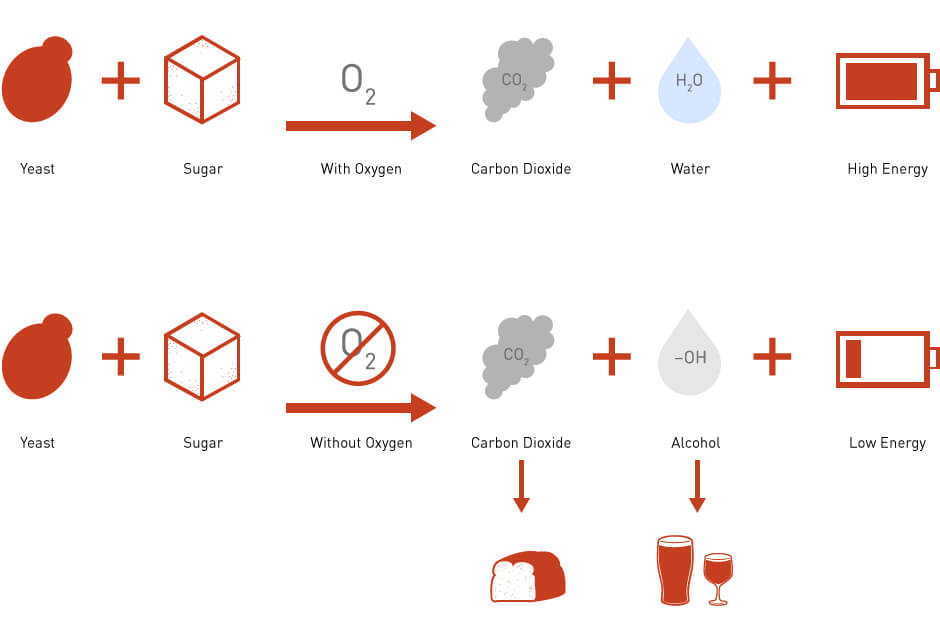
During the first two I began think about how to keep track of when to make the 2/3, & 1/3 sugar break nutrient additions. I had trouble inserting and removing the hydrometer (fat fingers) and the calculations with my refractometer are suspect. I did some quick calculations on the fermentation chemistry of sugar to CO2 and ethanol and realized that the CO2 produced is about 1/2 the weight of the sugars that are fermented. I should be able to detect this weight loss with my new 5 kilogram digital scale. From other sources I noted that honey is mostly sucrose and is about 90% fermentable. This coupled with a lot of information from the thread linked above I came up with the following plan.
Weigh all the ingredients and equipment at the start of fermentation.
Re-weigh the fermenter every day after de-gassing the mead.
Add additional nutrients (also weighed) at the calculated 2/3, & 1/3 sugar break weights.
Initial weights in grams.
Item --------WT ---- Total
Jug ------- 1381 - 1381
Honey ------ 1630 - 3011
Water ------ 2528 - 5539
Nutrients --- 0005 - 5544 <-- I will be using this as my initial weight. I lift the airlock prior to weighing.
Airlock ----- 0038 - 5582
The yeast weight is included with the water. I didn't get a separate weight for the yeast. I think I really only need the honey weight and the final weight to make use of this process.
1630 grams (~3.6#) of honey in 1 gallon gives a SG of 1.139. (A bit more that I wanted but the HBS got a little aggressive when filling my tubs with honey.) From Dr. Denards notes, Wyeast 1388 will ferment about 120 points of this or (120/139) * 1630 = 1407 grams of the honey. At 90% fermentability this means that 1407 * 0.9 = 1266 grams of sucrose will be converted to CO2 and ethanol.
Sucrose is C12H22O11 with a molecular weight of 342.3 grams/mole
4 molecules of CO2 will be produced for each of molecule of sucrose consumed by the yeast. The molecular weight of CO2 is 44 grams per mole so 4 * 44 = 176 grams of CO2 is produced for every 342.3 grams of sucrose. 176/342.3 = 0.51
Fermenting 1266 grams of sucrose will produce 0.51 * 1226 = 651 grams of CO2.
At the 2/3 sugar break, 1/3 of the sugar has been consumed and 1/3 of the total CO2 has been produced.
1/3 * 651 = 217 grams of CO2 produced. The fermenter should weigh 217 grams less at this point.
Similarly at the 1/3 break, 2/3's of the sugar has been consumed and 2/3's of the total CO2 has been produced.
2/3 * 651 = 434 grams of CO2 produced. The fermenter should weigh 434 grams less at this point.
Caveats:
I understand that I have made a few assumptions not limited to the following.
Honey is mostly sucrose and 90% fermentable by weight.
Any monosaccharides present that are fermented will throw off my 0.51 ratio of CO2 produced. Glucose gives a ratio of 0.49.
I can remove most/all the CO2 prior to weighing. (1 volume of CO2 per gallon weighs ~7 grams)
My 6 step weighing process is as follows.
So far I find this easier than having to sanitize, insert, read, and remove my hydrometer.
(Edit: 3-26-2019 These ratios/weights seem to be off in relation to when Bray makes his nutrient additions. See posts below for more details.)
Weights of fermenter for nutrient additions are:
2/3 : 5544 - 217 = 5327
1/3 : 5544 - 434 = 5110
Fermentation temp on counter is 68°F.
My data so far.
Date - Weight
02-20 - 5544
02-21 - 5523
02-22 - 5503
02-23 - out of town today
02-24 - 5416
02-25 - 5392 - getting close to first addition.
02-26 - 5368
02-27 - 5347
02-28 - 5324 - I added 3 grams of nutrients this morning.
02-28 - 5325 - After degassing and adding nutrients.
03-01 - 5296
03-02 - 5275
03-03 - 5262
03-04 - 5249
03-05 - 5238
03-06 - 5227
03-07 - 5216
03-08 - 5206
03-09 - 5196
03-10 - 5186
03-11 - 5177
03-12 - 5168
03-13 - 5159
03-14 - 5150
03-15 - 5142
03-16 - 5134
03-17 - 5127
03-18 - 5120
03-19 - 5113 - Second nutrient addition
03-20 - 5108
03-21 - 5106
03-22 - 5104
03-23 - 5102
03-24 - 5101
03-25 - 5100
Will update with additional weights over the next several weeks. * -
See this thread for the original recipe and many great notes on making a wonderful mead.
https://www.homebrewtalk.com/forum/threads/brays-one-month-mead.429241/
During the first two I began think about how to keep track of when to make the 2/3, & 1/3 sugar break nutrient additions. I had trouble inserting and removing the hydrometer (fat fingers) and the calculations with my refractometer are suspect. I did some quick calculations on the fermentation chemistry of sugar to CO2 and ethanol and realized that the CO2 produced is about 1/2 the weight of the sugars that are fermented. I should be able to detect this weight loss with my new 5 kilogram digital scale. From other sources I noted that honey is mostly sucrose and is about 90% fermentable. This coupled with a lot of information from the thread linked above I came up with the following plan.
Weigh all the ingredients and equipment at the start of fermentation.
Re-weigh the fermenter every day after de-gassing the mead.
Add additional nutrients (also weighed) at the calculated 2/3, & 1/3 sugar break weights.
Initial weights in grams.
Item --------WT ---- Total
Jug ------- 1381 - 1381
Honey ------ 1630 - 3011
Water ------ 2528 - 5539
Nutrients --- 0005 - 5544 <-- I will be using this as my initial weight. I lift the airlock prior to weighing.
Airlock ----- 0038 - 5582
The yeast weight is included with the water. I didn't get a separate weight for the yeast. I think I really only need the honey weight and the final weight to make use of this process.
1630 grams (~3.6#) of honey in 1 gallon gives a SG of 1.139. (A bit more that I wanted but the HBS got a little aggressive when filling my tubs with honey.) From Dr. Denards notes, Wyeast 1388 will ferment about 120 points of this or (120/139) * 1630 = 1407 grams of the honey. At 90% fermentability this means that 1407 * 0.9 = 1266 grams of sucrose will be converted to CO2 and ethanol.
Sucrose is C12H22O11 with a molecular weight of 342.3 grams/mole
4 molecules of CO2 will be produced for each of molecule of sucrose consumed by the yeast. The molecular weight of CO2 is 44 grams per mole so 4 * 44 = 176 grams of CO2 is produced for every 342.3 grams of sucrose. 176/342.3 = 0.51
Fermenting 1266 grams of sucrose will produce 0.51 * 1226 = 651 grams of CO2.
At the 2/3 sugar break, 1/3 of the sugar has been consumed and 1/3 of the total CO2 has been produced.
1/3 * 651 = 217 grams of CO2 produced. The fermenter should weigh 217 grams less at this point.
Similarly at the 1/3 break, 2/3's of the sugar has been consumed and 2/3's of the total CO2 has been produced.
2/3 * 651 = 434 grams of CO2 produced. The fermenter should weigh 434 grams less at this point.
Caveats:
I understand that I have made a few assumptions not limited to the following.
Honey is mostly sucrose and 90% fermentable by weight.
Any monosaccharides present that are fermented will throw off my 0.51 ratio of CO2 produced. Glucose gives a ratio of 0.49.
I can remove most/all the CO2 prior to weighing. (1 volume of CO2 per gallon weighs ~7 grams)
My 6 step weighing process is as follows.
- Turn on and tare the scale.
- Place fermenter on scale.
- Lift airlock.
- Record weight.
- Replace airlock.
- Return fermenter to counter.
So far I find this easier than having to sanitize, insert, read, and remove my hydrometer.
(Edit: 3-26-2019 These ratios/weights seem to be off in relation to when Bray makes his nutrient additions. See posts below for more details.)
Weights of fermenter for nutrient additions are:
2/3 : 5544 - 217 = 5327
1/3 : 5544 - 434 = 5110
Fermentation temp on counter is 68°F.
My data so far.
Date - Weight
02-20 - 5544
02-21 - 5523
02-22 - 5503
02-23 - out of town today
02-24 - 5416
02-25 - 5392 - getting close to first addition.
02-26 - 5368
02-27 - 5347
02-28 - 5324 - I added 3 grams of nutrients this morning.
02-28 - 5325 - After degassing and adding nutrients.
03-01 - 5296
03-02 - 5275
03-03 - 5262
03-04 - 5249
03-05 - 5238
03-06 - 5227
03-07 - 5216
03-08 - 5206
03-09 - 5196
03-10 - 5186
03-11 - 5177
03-12 - 5168
03-13 - 5159
03-14 - 5150
03-15 - 5142
03-16 - 5134
03-17 - 5127
03-18 - 5120
03-19 - 5113 - Second nutrient addition
03-20 - 5108
03-21 - 5106
03-22 - 5104
03-23 - 5102
03-24 - 5101
03-25 - 5100
Will update with additional weights over the next several weeks. * -
Last edited: