Last edited:


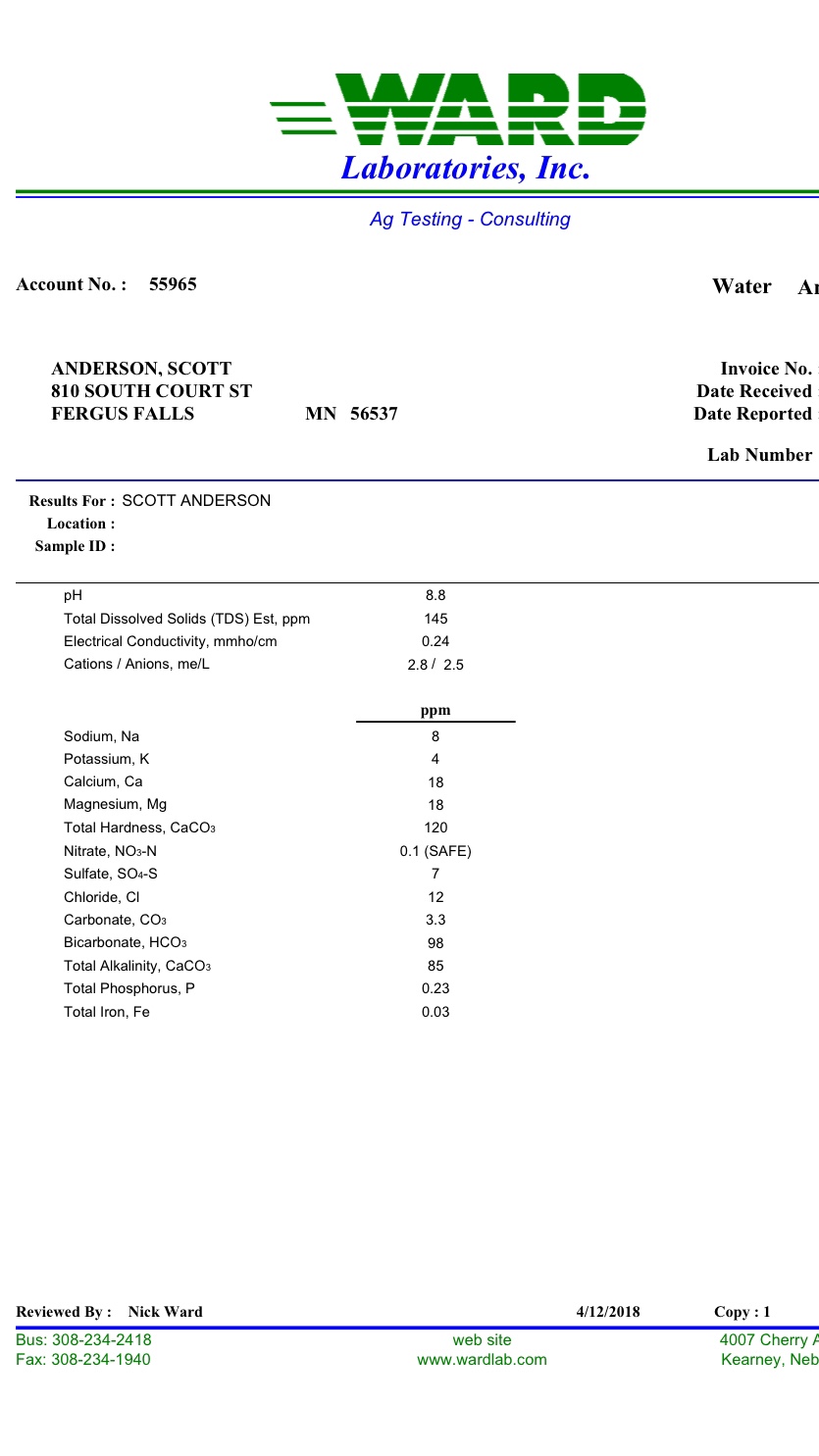
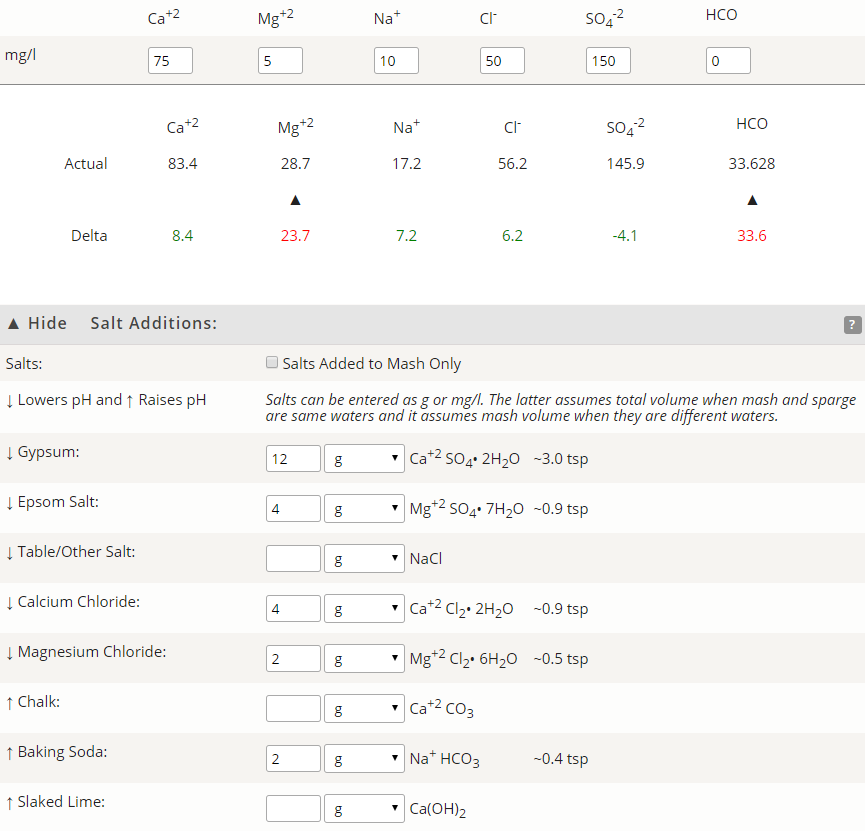
That is a pretty good water supply. The alkalinity level is well suited for brewing since it isn't too high and can easily be neutralized for brewing paler beers. As mentioned, Mg salts should probably be avoided since that level is near the upper end.
I've never heard of anyone using Tartaric acid for beer, it's normally for wine. It will work for pH adjustment, but may have a strong impact on beer flavour. I'm not sure.A 10 gallon recipe that is 21 lbs 2-row and 1lb carmel 40 and .75 lb carmel 60, I had also figured in 6g Tartaric acid that will bring ph to 5.4
That grain bill in distilled water will fall somewhere near 5.4 (it depends on the particular grain and batch, but it should be close), meaning you only have to counter the acid in your water.A 10 gallon recipe that is 21 lbs 2-row and 1lb carmel 40 and .75 lb carmel 60, I had also figured in 6g Tartaric acid that will bring ph to 5.4
My bad on the Tartaric acid, ill get some lactic acid coming, if that's the norm for a session IPA, but Im new to treating the water so I have stupid or redundant questions I apologize, alos I have a full volume recirculation system.
Also, I am trying to get good baseline for my water, my next brew will be one that I likely wont do again just because its the grains that I have left over and mixed up, but like I said I am new to this water chemistry game and am trying to wrap my head around what goes where and why, no I haven't read Palmers book on water..................yet.
Enter your email address to join: