Subject says it all. I realized my mistake as I was stirring the mash. I then crushed a tablet and added it. Mash is sitting now. I've had awful chlorophenol issues in the past and I'd rather dump now if it's too late. Any advice?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Quick Advice Needed! Forgot to add Campden tab until after I mashed in
- Thread starter reuliss
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
I'm guessing it's too late but I wouldn't dump it for that alone. I've had similar issues with chlorines and chloramines and now religiously prepare water 24 hours ahead of mash using a carbon filter and a quarter crushed Campden tablet per 5 gallon batch.
ChrisfromAbby
Well-Known Member
It'll work fine. You just need to prevent the yeast from getting a chance to chew on the chloramine.
btbnl
Well-Known Member
It'll work fine. You just need to prevent the yeast from getting a chance to chew on the chloramine.
But chlorophenols come from the reaction between the chloramine and the phenols in the wort, no?
I did the same thing with my 3rd batch last brew day. I just added the campden tablet half way through the mash (when I took a pH sample) and didn't have a problem.
ChrisfromAbby
Well-Known Member
I always understood it as a metabolic issue.
http://beer-geeking.blogspot.ca/2011/05/water-chlorine-chloramine-and.html
Chris
http://beer-geeking.blogspot.ca/2011/05/water-chlorine-chloramine-and.html
Chris
But chlorophenols come from the reaction between the chloramine and the phenols in the wort, no?
I did the same thing with my 3rd batch last brew day. I just added the campden tablet half way through the mash (when I took a pH sample) and didn't have a problem.
This is correct which is why I'm worried. The problem is that you don't know if you have an issue until the beer is done and carbed up. Im hoping AJ or Martin are lurking.
I always understood it as a metabolic issue.
http://beer-geeking.blogspot.ca/2011/05/water-chlorine-chloramine-and.html
Chris
I've read that link before. Author definitely has it wrong.
btbnl
Well-Known Member
This is correct which is why I'm worried. The problem is that you don't know if you have an issue until the beer is done and carbed up. Im hoping AJ or Martin are lurking.
My understanding is that they can form any time phenols are present (and there are certainly yeasts that produce more phenols), but if they had formed during the mash then you'd be able to taste them immediately wouldn't you?
This looks like what we need, but it's behind a paywall.
Anytime chlorine or chloramine and phenol are present the potential for formation of chlorphenolics is there and malt contains phenols so you clearly do not want chloramine carrying water to contact your mash at any time. Nor do you want it to contact your finished wort.
Lots of brewers have water with finite chloramine content and get away with it but no one should assume that he will. If you forget to treat the water I suppose the next best thing is to add the campden tablet, dissolved in a reasonable volume of water, to the mash and mix it in very thoroughly. If the kinetics of the chlophenolic forming reaction are as slow as other mash reactions I suppose there is a chance that you would get fewer chlorphenolics than if you did nothing and perhaps have levels beneath detection. In any case you should keep the beer and see if you are one of the lucky ones.
Lots of brewers have water with finite chloramine content and get away with it but no one should assume that he will. If you forget to treat the water I suppose the next best thing is to add the campden tablet, dissolved in a reasonable volume of water, to the mash and mix it in very thoroughly. If the kinetics of the chlophenolic forming reaction are as slow as other mash reactions I suppose there is a chance that you would get fewer chlorphenolics than if you did nothing and perhaps have levels beneath detection. In any case you should keep the beer and see if you are one of the lucky ones.
ChrisfromAbby
Well-Known Member
This sounds like a good XBEERiment for Brulosophy, to compare metabisulfite additions to the HLT and the mash tun.
In the last 30 years I've brewed using spring water, well water, triple osmosis water, and tap water. I honestly don't think the difference between them was as much as when a batch of mead got a bad Brett species. :-( Mind you, our water here is very pure. Low mineral content and little contamination so the chloramine levels are fairly low so you may have greater influence. Also depend on what you are brewing I suppose - a Czech Pils being more delicate than a Russian Imperial Stout.
But I agree. If phenols in the wort are reacting with the chloramines then you should taste it in the wort or of the mash tun. I however understood that it was POLYphenols produced by the yeast tasting with the chloramines metabolically.
In the last 30 years I've brewed using spring water, well water, triple osmosis water, and tap water. I honestly don't think the difference between them was as much as when a batch of mead got a bad Brett species. :-( Mind you, our water here is very pure. Low mineral content and little contamination so the chloramine levels are fairly low so you may have greater influence. Also depend on what you are brewing I suppose - a Czech Pils being more delicate than a Russian Imperial Stout.
But I agree. If phenols in the wort are reacting with the chloramines then you should taste it in the wort or of the mash tun. I however understood that it was POLYphenols produced by the yeast tasting with the chloramines metabolically.
btbnl
Well-Known Member
This looks like what we need, but it's behind a paywall.
Turns out my lab library has a subscription that gets me behind the paywall.
Being Belgian brewers, the authors are actually looking to optimize the production of ferulic acid (FA) as a phenol precursor. They investigate the impact of mash temperature, pH, duration, thickness, grist coarseness and composition, and stirring regime.
Temperature: Free FA production peaks at 40C/104F before dropping 70% to a flat minimum above 60C/140F.
pH: Free FA production peaks at pH 5.8, dropping by 25% in the pH 5.2-5.6 range of most interest to us.
Duration: A 30 minute mash reduced the free FA production by 25%; a 2+ hour mash increased it by 50%.
Thickness: Free FA production was maximized at medium density (quoted as 10 degrees Plato) dropping by up to 10% at higher or lower densities.
Grist coarseness: Free FA production was reduced by 25% when changing the grinding disk separation from 0.1mm to 2mm.
Composition: Baseline results were from pure pilsner malt; replacing 50% of the grain bill with adjuncts typically halved free FA production, except with wheat and rye, and Vienna and Munich malts.
Stirring: Continuous stirring increased free FA production by 50% compared with an unstirred mash.
Their conclusions were that free FA could be maximized by including a low temperature rest (40C) during the mash, keeping the pH relatively high (5.8), and stirring a lot.
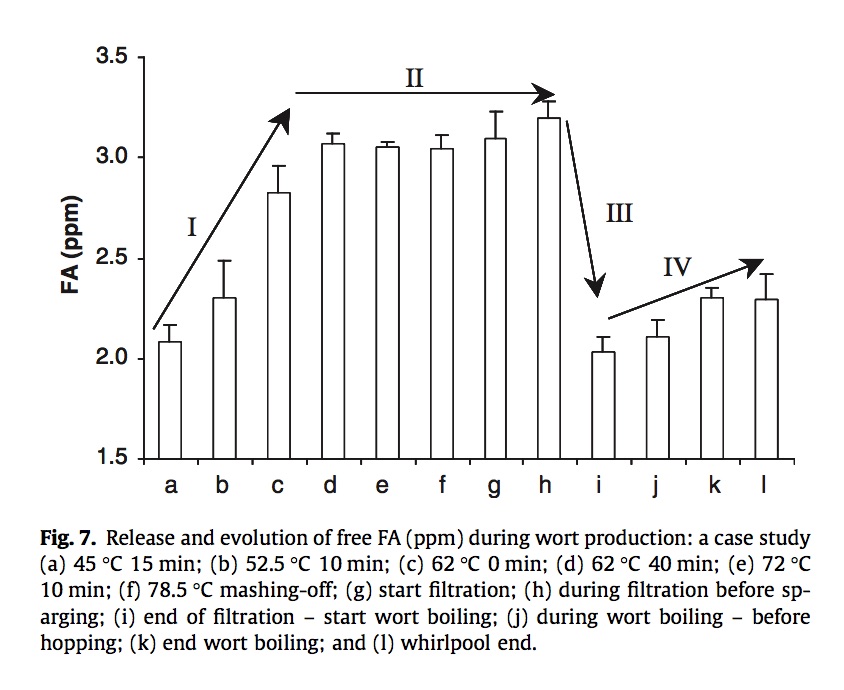
They then set up a full-scale test brew and measured free FA concentrations throughout. Note that in the figure below the scale starts at 1.5ppm, somewhat disguising the fact that most of the production still comes from step (a) - the initial low-T rest - with the remainder coming from the full duration of the mash - to step (d). Sparging reduces the concentration but not the total content, while boiling increases the concentration but reduces the content due to thermal decarboxylation. Finally hop additions increase free FA by ~10%.
If you're interested in the full paper, pm me.
Similar threads
- Replies
- 4
- Views
- 762
- Replies
- 39
- Views
- 6K
- Replies
- 512
- Views
- 20K

