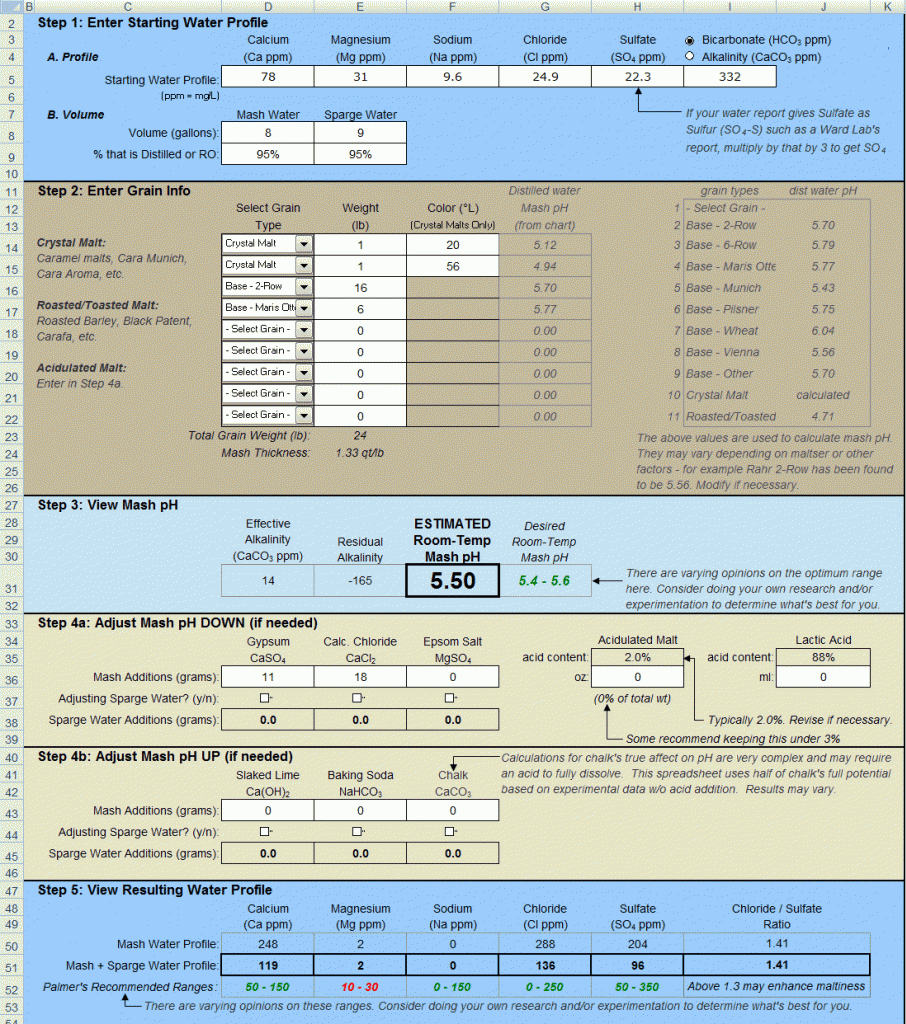
Hi all - I've been trying to get the land of pH in my brewing process dialed in. Using a new and freshly calibrated sensor on my pH56, I'm getting a mash pH of 5.25@70F after 45 minutes of mashing. My target mash pH is around 5.5@70F via the water spreadsheet 3.0, so I'm surprised I missed it by this much. Below is what my recipe looks like - according to the spreadsheet my mineral additions into RO water look right?
Style: American Pale Ale
TYPE: All Grain
Taste: (35.0)
Recipe Specifications
--------------------------
Boil Size: 14.35 gal
Post Boil Volume: 12.48 gal
Batch Size (fermenter): 12.00 gal
Bottling Volume: 12.00 gal
Estimated OG: 13.308 Plato
Estimated Color: 7.5 SRM
Estimated IBU: 39.5 IBUs
Brewhouse Efficiency: 75.00 %
Est Mash Efficiency: 75.0 %
Boil Time: 90 Minutes
Ingredients:
------------
Amt Name Type # %/IBU
18.00 g Calcium Chloride (Mash 60.0 mins) Water Agent 1 -
11.00 g Gypsum (Calcium Sulfate) (Mash 60.0 mins Water Agent 2 -
21 lbs Pale Malt (2 Row) US (2.0 SRM) Grain 3 87.5 %
1 lbs Biscuit Malt (23.0 SRM) Grain 4 4.2 %
1 lbs Cara-Pils/Dextrine (2.0 SRM) Grain 5 4.2 %
1 lbs Caramel/Crystal Malt - 60L (60.0 SRM) Grain 6 4.2 %
1.50 oz Cascade [7.30 %] - First Wort 90.0 min Hop 7 21.2 IBUs
1.00 oz Centennial [11.10 %] - Boil 60.0 min Hop 8 18.3 IBUs
1.50 oz Citra [14.10 %] - Steep/Whirlpool 0.0 m Hop 9 0.0 IBUs
1.0 pkg Safale American (DCL/Fermentis #US-05) Yeast 10 -
Mash Schedule: My Mash
Total Grain Weight: 24 lbs
----------------------------
Name Description Step Temperat Step Time
Step Add 31.99 qt of water at 165.1 F 152.0 F 40 min
Style: American Pale Ale
TYPE: All Grain
Taste: (35.0)
Recipe Specifications
--------------------------
Boil Size: 14.35 gal
Post Boil Volume: 12.48 gal
Batch Size (fermenter): 12.00 gal
Bottling Volume: 12.00 gal
Estimated OG: 13.308 Plato
Estimated Color: 7.5 SRM
Estimated IBU: 39.5 IBUs
Brewhouse Efficiency: 75.00 %
Est Mash Efficiency: 75.0 %
Boil Time: 90 Minutes
Ingredients:
------------
Amt Name Type # %/IBU
18.00 g Calcium Chloride (Mash 60.0 mins) Water Agent 1 -
11.00 g Gypsum (Calcium Sulfate) (Mash 60.0 mins Water Agent 2 -
21 lbs Pale Malt (2 Row) US (2.0 SRM) Grain 3 87.5 %
1 lbs Biscuit Malt (23.0 SRM) Grain 4 4.2 %
1 lbs Cara-Pils/Dextrine (2.0 SRM) Grain 5 4.2 %
1 lbs Caramel/Crystal Malt - 60L (60.0 SRM) Grain 6 4.2 %
1.50 oz Cascade [7.30 %] - First Wort 90.0 min Hop 7 21.2 IBUs
1.00 oz Centennial [11.10 %] - Boil 60.0 min Hop 8 18.3 IBUs
1.50 oz Citra [14.10 %] - Steep/Whirlpool 0.0 m Hop 9 0.0 IBUs
1.0 pkg Safale American (DCL/Fermentis #US-05) Yeast 10 -
Mash Schedule: My Mash
Total Grain Weight: 24 lbs
----------------------------
Name Description Step Temperat Step Time
Step Add 31.99 qt of water at 165.1 F 152.0 F 40 min