I did my first winter brew on Friday and had a bit of a snafu. If I'd known how long it would take to chill I'd have made a mid brew change to the recipe. I had ordered a pond pump from amazon prime that was supposed to arrive Thursday but got delayed. Mail usually comes by noon so I timed my brew to finish the boil around 1pm. We had a big snowstorm so the mail didn't arrive until 2:30.
TL;DR:
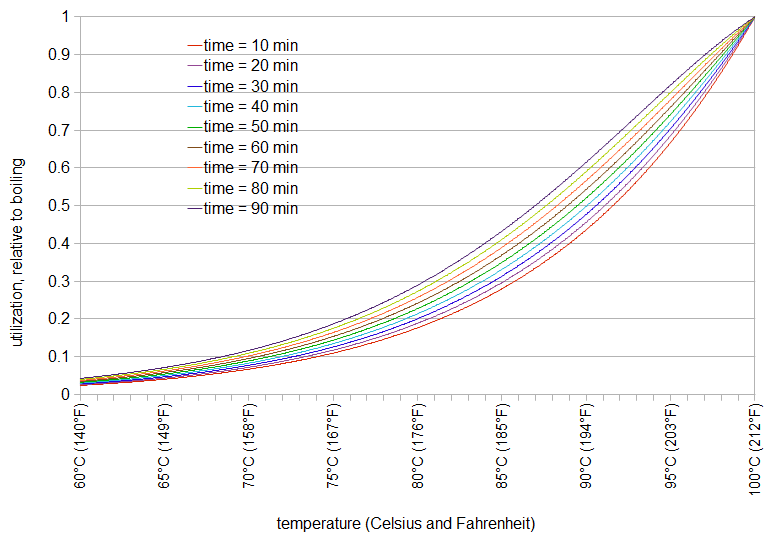
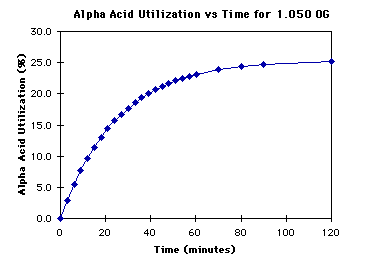
Chilling my beer to 170 took way longer than expected. I had a large 5 minute hop addition and another large addition at FO. With the slowed chill, those hops were at high enough temps to isomerize longer than planned. I changed my recipe making those additions boil additions for the entire duration they were above 180. That shows an IBU of 160. Will it really be that bitter or does the rate of isomerization drop with the temp?
This beer will be much more bitter than planned, but will it be that bad?
The good news is that once the pump arrived, it worked very well.
TL;DR:
Chilling my beer to 170 took way longer than expected. I had a large 5 minute hop addition and another large addition at FO. With the slowed chill, those hops were at high enough temps to isomerize longer than planned. I changed my recipe making those additions boil additions for the entire duration they were above 180. That shows an IBU of 160. Will it really be that bitter or does the rate of isomerization drop with the temp?
This beer will be much more bitter than planned, but will it be that bad?
The good news is that once the pump arrived, it worked very well.