luizffgarcia
Well-Known Member
- Joined
- Jan 26, 2016
- Messages
- 199
- Reaction score
- 20
Hi guys,
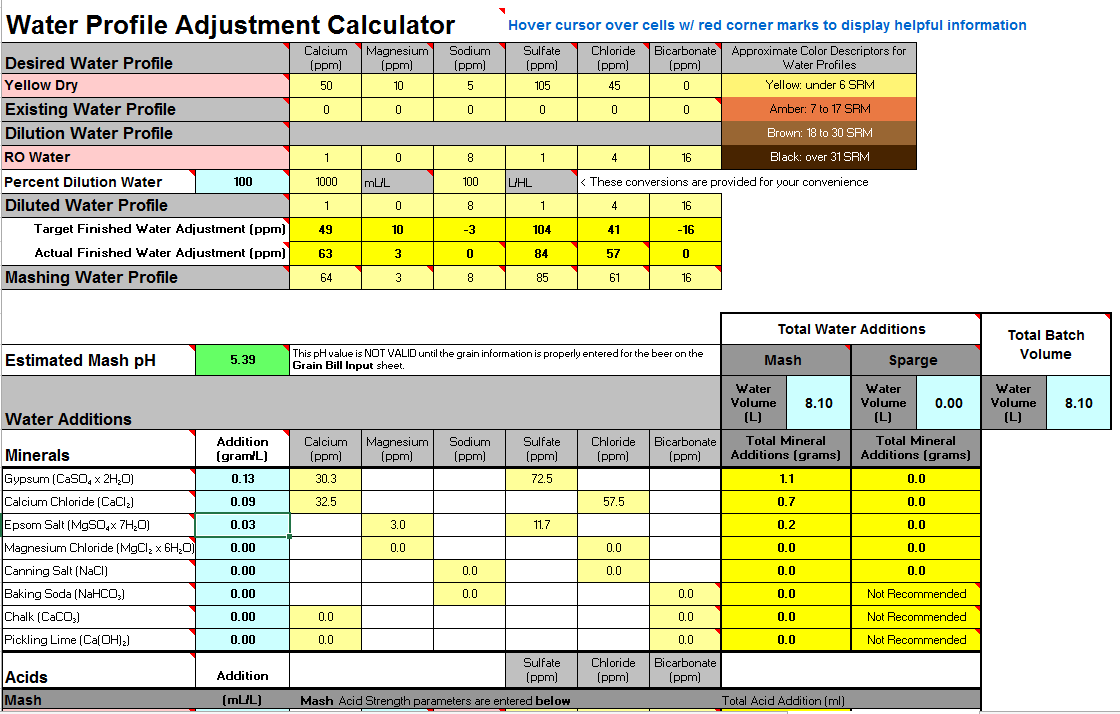
I have been trying to better understand my water, which i build from 100% distilled and there is one aspect i am worried about, the buffer.
If you look at the screenshot the alkalinity is 13, and reading thru the example profiles i see this is very low comparing to others.
I just purchased a MW102 to better control my mash Ph, but i need to understand what is the alkalinity role in this.
So, does this water look ok for a APA? Is the buffer to low for some reason? And if yes, how can i correct it?
I mostly brew APAs and IPAs, for my IPAs the only change is i raise the Sulfate to around 220ppm, still get low alkalinity when i do that.
Thanks for the help
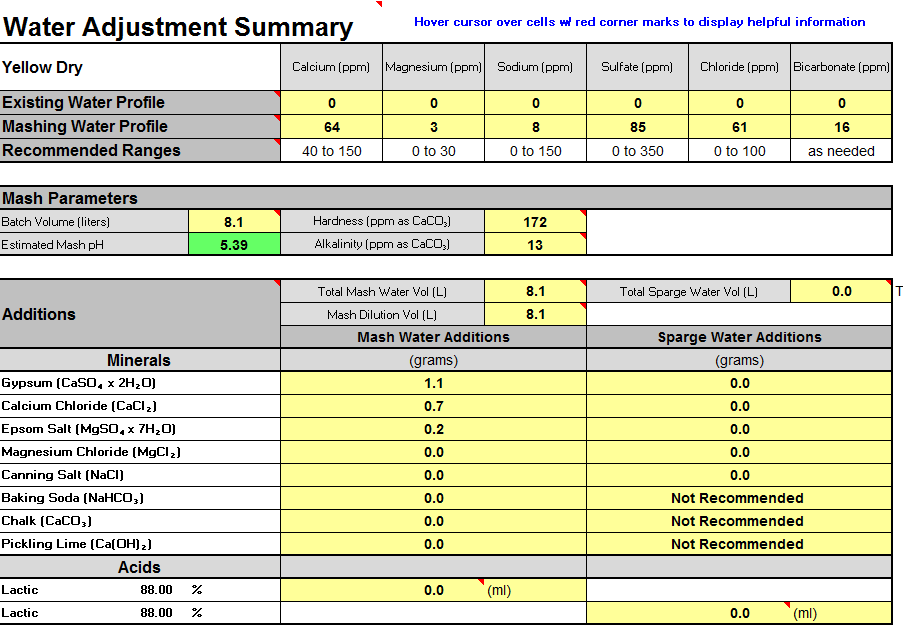
I have been trying to better understand my water, which i build from 100% distilled and there is one aspect i am worried about, the buffer.
If you look at the screenshot the alkalinity is 13, and reading thru the example profiles i see this is very low comparing to others.
I just purchased a MW102 to better control my mash Ph, but i need to understand what is the alkalinity role in this.
So, does this water look ok for a APA? Is the buffer to low for some reason? And if yes, how can i correct it?
I mostly brew APAs and IPAs, for my IPAs the only change is i raise the Sulfate to around 220ppm, still get low alkalinity when i do that.
Thanks for the help