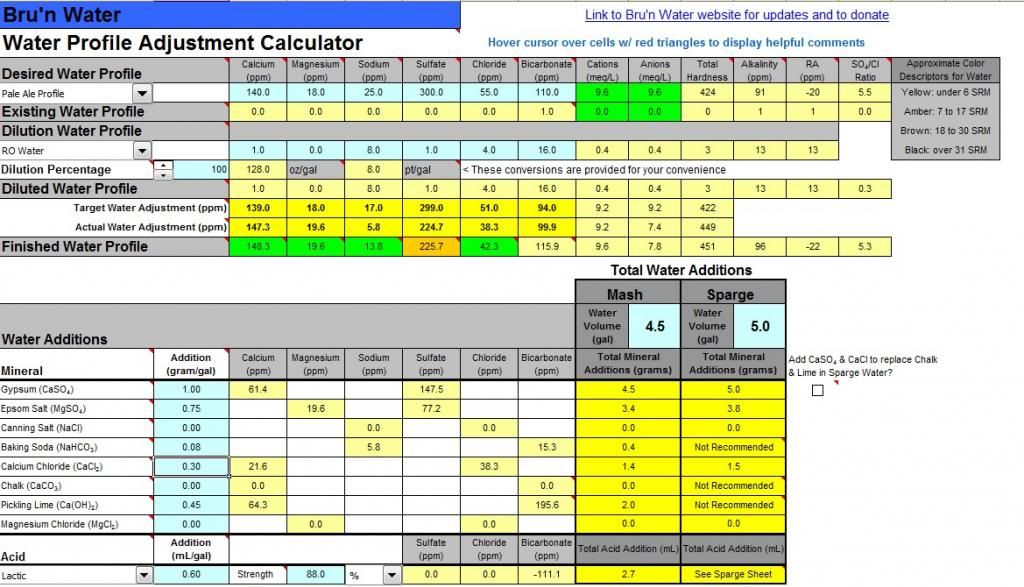
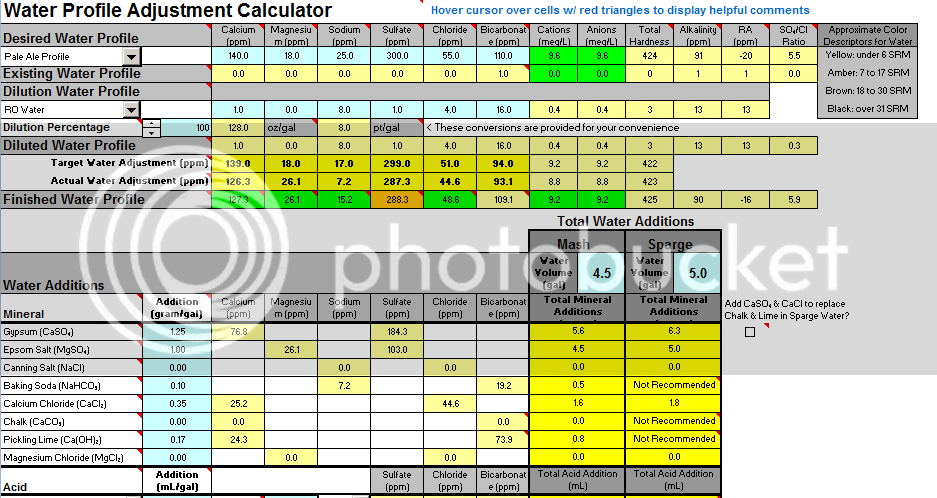
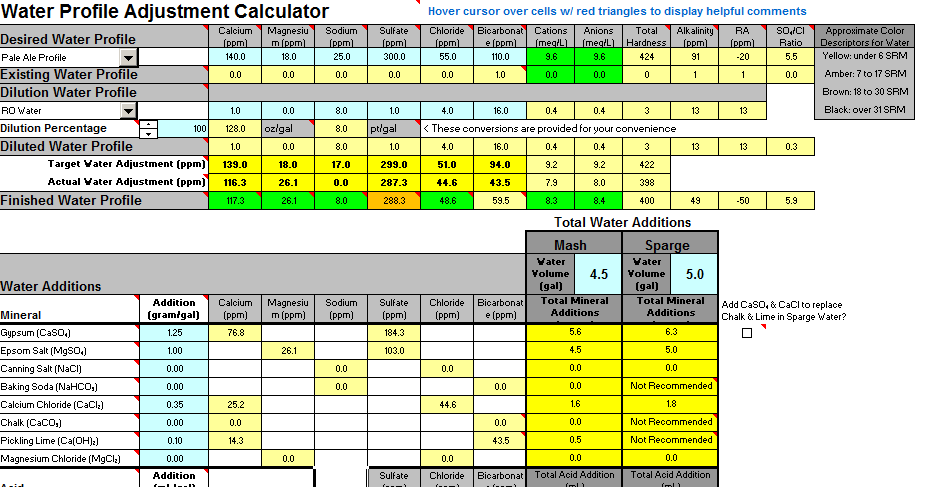
Hey Guys I am brewing an IPA using my first water profile adjustment and wanted some feedback. I plan to use 100% RO water for my base and add additions to my water. My estimated room pH is at 5.5, but just wanted to confirm my levels of additions are OK - would you change anything or does this look OK? Only thing I notice is that my anion level is a little lower than suggested. Thanks in advance for your feedback!