Lord_Lycoperdon
New Member
I was talking to guy who sells kombucha here in Oakland about how he bottles it. He just uses glass bottles with plastic lids. and that the pressure stops the fermentation and that you don't have to worry about it with kombucha.
Is this guy right? Is the same true about beer? Has anyone ever even had a bottle explode? (I never have...)
I searched the internet for answers and I found this article (below) but it is kinda confusing and I'm not sure how much pressure is in a bottle of beer~
Titre du document / Document title
How does yeast respond to pressure?
Auteur(s) / Author(s)
FERNANDES P. M. B. ;
Résumé / Abstract
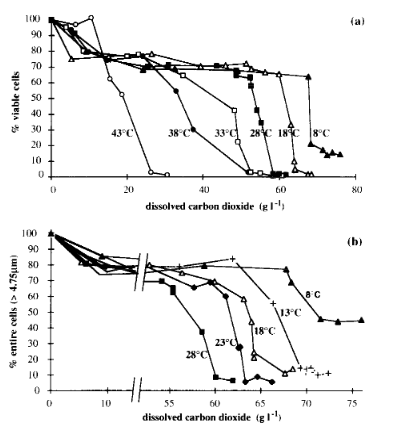
The brewing and baking yeast Saccharomyces cerevisiae has been used as a model for stress response studies of eukaryotic cells. In this review we focus on the effect of high hydrostatic pressure (HHP) on S. cerevisiae. HHP exerts a broad effect on yeast cells characteristic of common stresses, mainly associated with protein alteration and lipid bilayer phase transition. Like most stresses, pressure induces cell cycle arrest. Below 50 MPa (500 atm) yeast cell morphology is unaffected whereas above 220 MPa wild-type cells are killed. S. cerevisiae cells can acquire barotolerance if they are pretreated with a sublethal stress due to temperature, ethanol, hydrogen peroxide, or pressure. Nevertheless, pressure only leads to protection against severe stress if, after pressure pretreatment, the cells are also re-incubated at room pressure. We attribute this effect to the inhibition of the protein synthesis apparatus under HHP. The global genome expression analysis of S. cerevisiae cells submitted to HHP revealed a stress response profile. The majority of the up-regulated genes are involved in stress defense and carbohydrate metabolism while most repressed genes belong to the cell cycle progression and protein synthesis categories. However, the signaling pathway involved in the pressure response is still to be elucidated. Nitric oxide, a signaling molecule involved in the regulation of a large number of cellular functions, confers baroprotection. Furthermore, S. cerevisiae cells in the early exponential phase submitted to 50-MPa pressure show induction of the expression level of the nitric oxide synthase inducible isoform. As pressure becomes an important biotechnological tool, studies concerning this kind of stress in microorganisms are imperative.
Revue / Journal Title
Brazilian journal of medical and biological research ISSN 0100-879X CODEN BJMRDK
Source / Source
2005, vol. 38, no8, pp. 1239-1245 [7 page(s) (article)]
Langue / Language
Anglais
Editeur / Publisher
Associação Brasileira de Divulgação Científica, Ribeirão Preto, BRESIL (1981) (Revue)
Localisation / Location
INIST-CNRS, Cote INIST : 14110, 35400013239836.0120
Is this guy right? Is the same true about beer? Has anyone ever even had a bottle explode? (I never have...)
I searched the internet for answers and I found this article (below) but it is kinda confusing and I'm not sure how much pressure is in a bottle of beer~
Titre du document / Document title
How does yeast respond to pressure?
Auteur(s) / Author(s)
FERNANDES P. M. B. ;
Résumé / Abstract
The brewing and baking yeast Saccharomyces cerevisiae has been used as a model for stress response studies of eukaryotic cells. In this review we focus on the effect of high hydrostatic pressure (HHP) on S. cerevisiae. HHP exerts a broad effect on yeast cells characteristic of common stresses, mainly associated with protein alteration and lipid bilayer phase transition. Like most stresses, pressure induces cell cycle arrest. Below 50 MPa (500 atm) yeast cell morphology is unaffected whereas above 220 MPa wild-type cells are killed. S. cerevisiae cells can acquire barotolerance if they are pretreated with a sublethal stress due to temperature, ethanol, hydrogen peroxide, or pressure. Nevertheless, pressure only leads to protection against severe stress if, after pressure pretreatment, the cells are also re-incubated at room pressure. We attribute this effect to the inhibition of the protein synthesis apparatus under HHP. The global genome expression analysis of S. cerevisiae cells submitted to HHP revealed a stress response profile. The majority of the up-regulated genes are involved in stress defense and carbohydrate metabolism while most repressed genes belong to the cell cycle progression and protein synthesis categories. However, the signaling pathway involved in the pressure response is still to be elucidated. Nitric oxide, a signaling molecule involved in the regulation of a large number of cellular functions, confers baroprotection. Furthermore, S. cerevisiae cells in the early exponential phase submitted to 50-MPa pressure show induction of the expression level of the nitric oxide synthase inducible isoform. As pressure becomes an important biotechnological tool, studies concerning this kind of stress in microorganisms are imperative.
Revue / Journal Title
Brazilian journal of medical and biological research ISSN 0100-879X CODEN BJMRDK
Source / Source
2005, vol. 38, no8, pp. 1239-1245 [7 page(s) (article)]
Langue / Language
Anglais
Editeur / Publisher
Associação Brasileira de Divulgação Científica, Ribeirão Preto, BRESIL (1981) (Revue)
Localisation / Location
INIST-CNRS, Cote INIST : 14110, 35400013239836.0120