John Meerse
Member
- Joined
- Apr 7, 2019
- Messages
- 11
- Reaction score
- 2
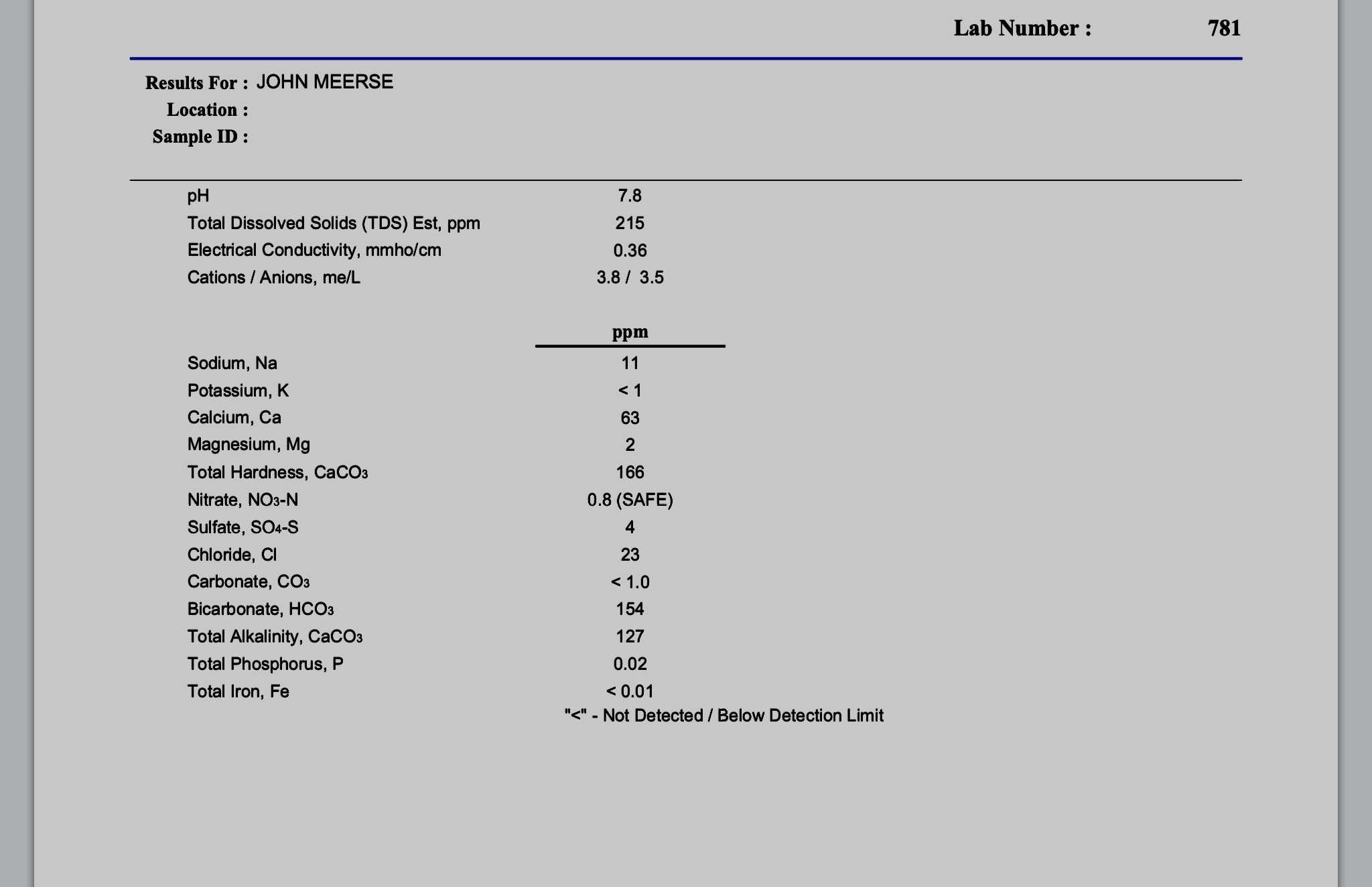
I’m planning on following A.J. deLange’s method for using slaked lime to decarbonate my water, as outlined in the Water book by Palmer and Kaminski. I’ve run through the process a couple times with small amounts of water (1 pint and 3 quarts) and feel confident I understand the process. I’m going to brew this Sunday, and so will treat my water on Saturday to allow time for precipitation to occur.
I have 2 questions: If I need 9 gallons for my mash water, how much should I treat, since I’ll be leaving some behind with the precipitate in it?
Do I need to also treat my sparge water, or is it sufficient to just treat my strike water?
I’ve been using lactic acid to neutralize the alkalinity, but I think I’m getting off flavors in the finished beer (typically about 1.25 tablespoons). I don’t have phosphoric acid, and I believe the amount of acid malt I’d need would go over the recommended limit. I don’t have access to RO water, and really don’t want to buy that much distilled water and have to recycle all those jugs.
Thanks very much!
I have 2 questions: If I need 9 gallons for my mash water, how much should I treat, since I’ll be leaving some behind with the precipitate in it?
Do I need to also treat my sparge water, or is it sufficient to just treat my strike water?
I’ve been using lactic acid to neutralize the alkalinity, but I think I’m getting off flavors in the finished beer (typically about 1.25 tablespoons). I don’t have phosphoric acid, and I believe the amount of acid malt I’d need would go over the recommended limit. I don’t have access to RO water, and really don’t want to buy that much distilled water and have to recycle all those jugs.
Thanks very much!














































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
