Serious question AJ - do you have Asperger Syndrome?
I'm hardly qualified to judge but yes, I rather think I am so blessed. A lifetime of ignoring what is now called 'political correctness' and, in general, wondering what all the fuss is about (e.g. this post) has brought me more success in life than I had ever hoped for. I did see once an article in which the author was trying to quantify the 'suffering' of Asperger's only to find that the victims of this 'malady' unanimously consider it a gift. I certainly do and that's why I think I may qualify. I m definitely of the opinion that the Asperger's-ish guys (remember that I was an engineer and so know many, many of them) have got it right and that the world would operate a lot more smoothly if people thought the way we do. There is no question that this personality trait defines an eccentric and so I definitely plead guilty to that label if you need to go labeling people.
You are obviously one of the most knowledgeable people around here when it comes to water chemistry,
Thanks. But see Dilbert's 'The Knack' on YouTube
..but the way you consistently imply that people who disagree with you lack common sense...
We both know that common sense is extremely rare these day(much rarer today than it was in the past). Nevertheless I do often appeal to common sense because it is very powerful. If common sense dictates that you can't produce a product to a high quality standard in a country half way around the world and ship it to a consumer in the US for $10 (unless it's toothpicks or chop sticks or something like that) and someone thinks that you can and you advise that person to use his common sense you are obviously implying that that person has not used his common sense but you are not implying that he has none. You will note that the posts do not say 'You have no common sense'. They say things like 'Common sense should tell you' or 'Use common sense.' If you realize that you have put your common sense on hold and, as a result of such a comment, get it down and use it then you have benefited. If your feelings got hurt, well that's "stiff bikkies" as the Aussies say. You have still benefited. In the olden days shaming a student for failure to think or use common sense was a common pedagogical technique and it worked. The current policies in which teachers are directed to put the student's self esteem before his intellectual progress is responsible for the appalling state of public education in this country.
...and intelligence make me wonder.
Let's face it: 90% of the population is in the bottom 9 deciles. I can't do anything about that except hope that some 'education' (what I try to provide here) will help them understand their world (brewing world anyway) a little better. I have never used phrases like "That's stupid" or "You are stupid" in a post. While lots of stupid statements are made here usually the post is the result of ignorance rather than stupidity, You, for example, seem to be ignorant of basic statistical knowledge. OK. So what? Perhaps you are a brilliant poet or musician. It doesn't matter. I can try to give you some insight into the statistical methods you need to understand if not lay out the whole necessary nine yards. If that makes you feel stupid, it shouldn't. It should make you feel ignorant and resolved to remove that ignorance. If your feelings get hurt then that's your problem - not mine. My intent is to help you understand the science (this is a science forum), not boost your self esteem.
.I've even seen you rudely snap at Martin Brungard.
Wasn't aware Martin had protected status here.
I'm just trying to put your replies into perspective here.
To help me help you let's consider a recent sequence
I cannot seem to find anywhere either on my PC software (beersmith) or the other calculators of how much my water pH will be reduced without adding grain and just adding salt.
Oh, come on. Don't toy with the person.
Yes, adding calcium salts like gypsum or calcium chloride won't change the pH of WATER, but they do change the pH of the wort in a mash.
To which I responded "Que?"
Would that be an example of me being rude to Martin or Martin being rude to me?
I adamantly disagree that you can draw any firm conclusions on a device's performance based on Amazon reviews.
For there to be a disagreement, adamant or otherwise, you would have to find where I said that you could draw firm conclusions and you won'y be able to do that. A basic understanding of freshman statistics says that you will probably get a more realistic picture that way than from your observations of 2 meters though and that's why I recommend people do that. Common sense also tells you the shortcomings of the Amazon method.
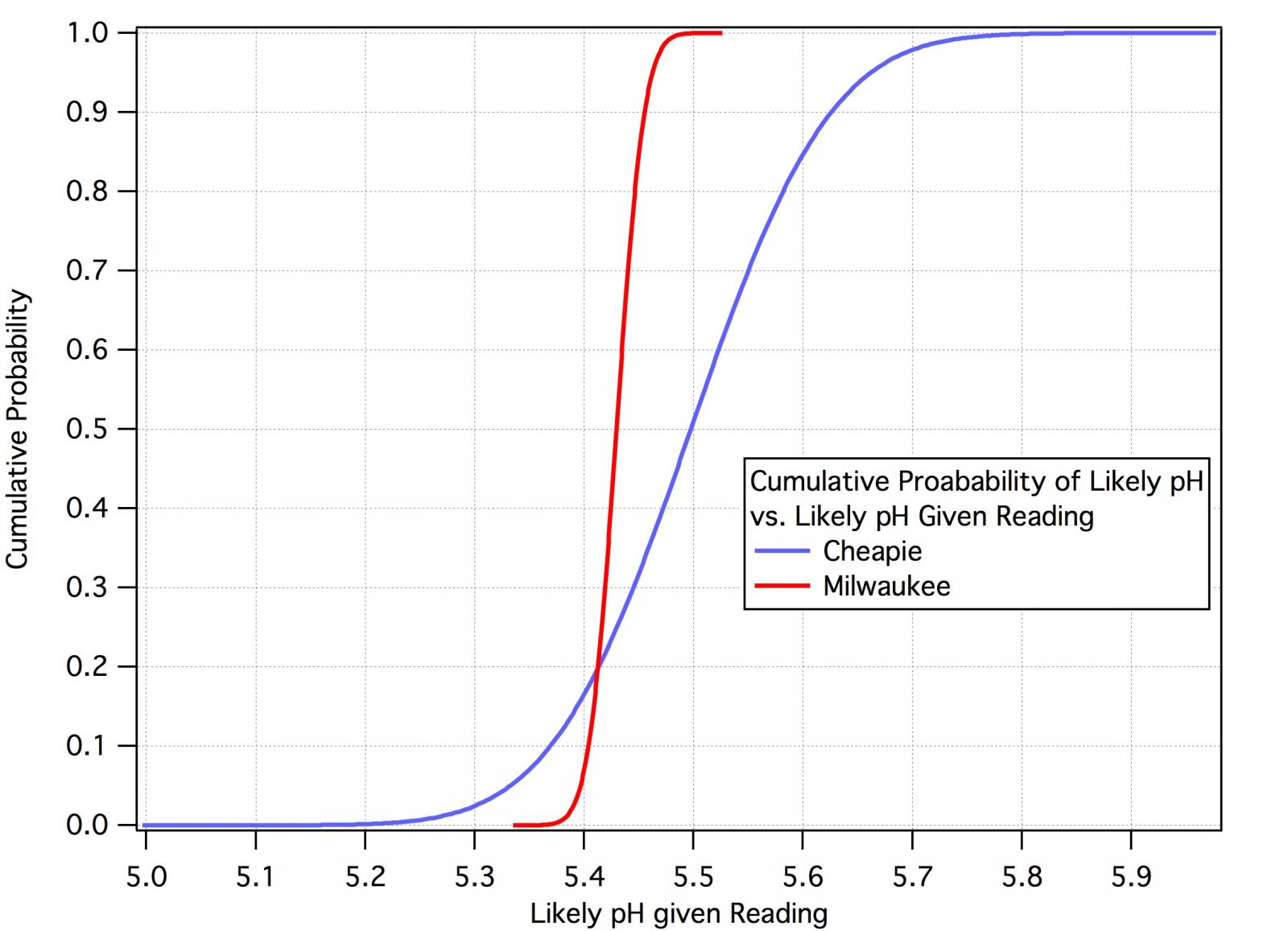
As far as determining if these meters meet their specs, with enough measurements I should very clearly be able to determine if their standard deviation is less than 0.1.
You can determine whether the rmse of the readings from the two meters is less than 0.1 but you cannot determine the accuracy of the meters. To do that you would need a more accurate meter to compare your meter's readings to.
That's the point of my ongoing tests. This isn't a peer reviewed white paper, this is a transparent ongoing homebrew experiment. After a while, if it turns out that these meters are pure crap after XX months, or if they are rock solid, I will attempt to draw a conclusion and quantify the results.
You need to get a basic statistics text and learn enough to be able to 'design' your experiment(s). A big part of this is determining the sample size that will give you results that carry reasonable statistical confidence. You will quickly discover that you won't get that with n = 2.
The jury is still out in my opinion...
That's because your thinking is ruled by bias. Try to free yourself of this and see things as they are. Even if the meter does meet it's spec of 0.1 rmse that is not sufficient for most people who use a pH meter in brewing. You can;t see, for example, if an acid addition gives you the desired 0.05 decrease in mash pH in a case where that's what you want.