No pH prediction software is very good. It cannot be very good unless you have good model for malts and good data to put into it. The good model exists and has been published here but none of the programs I am aware of use it and those that come closest don't have good data. To get good data you would have to make a laborious set of measurements on each malt and process the data from those measurements using techniques unfamiliar to many. It is much easier to simply do a test mash. Many of the programs are good enough to give you an idea as to how a test mash is likely to go an this, plus their ability to give you insight into "what if" situations and thus educate the user on the wiles of mash behaviour is their greatest value.
Bru'n water suffers (or suffered - don't know if the problem has been fixed) from a problem in which the volume of the mash water was improperly interpreted. It is known to give wild answers in some cases especially where dark malts are used. In fact that is the case for all the programs. It doesn't matter if you mismodel paler malts so much because there DI pH's are close to the desired mash pH. With the dark malts it is more distant and more variable and the malt model needs to accurately reflect this. The good news is these malts are used in relatively small quantity.
So you have one program that tells you mash pH is likely to be 4.59, which is clearly way out of the ball park, one that assures you that it will be 5.03, which is possible but unlikely, and a handful of others that tell you 5.4. To add to your confusion I'll toss in another estimate from a robust sheet (i.e. one that uses the proper models for everything) and that is 5.54. Now while the program that came up with that estimate does all the math right it doesn't have the "right" numbers to put into the formuals. I don't know that the chocolate malt that I did the detailed measurements on is anything but approximately close in its properties to the chocolate malt that you are going to use.
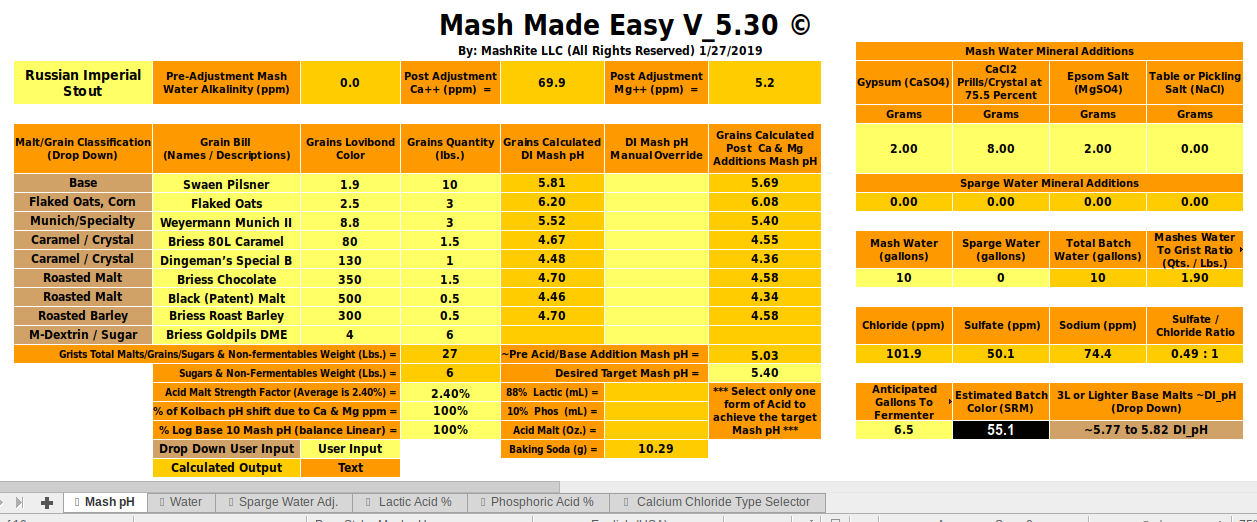
Here's the proton deficit breakdown for the estimate:
Now, as stated, we can't assert that the mash pH you will experience will be 5.54 because we don't know your malts we can determine that it will probably be something in the vicinity of 5.54 and do a test mash to see if we do indeed get that and, in my experience, you will because you have only 13% black malts.
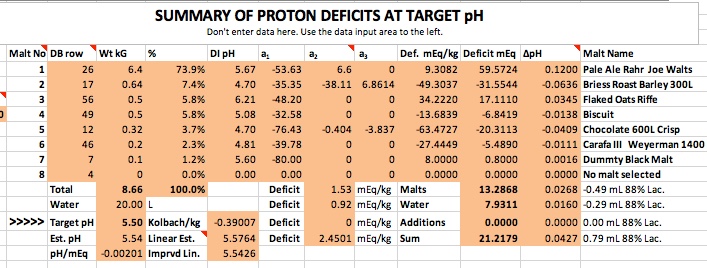
As an example of the "what if" use of spreadsheets we can ask "What if the pH were actually 5.03? How much extra black malt would it take to get there? The proton deficit story for a pH of 5.03 is in this picture:
The first thing we see is that it takes a lot of acid to get this pale ale malt (Rahr) to pH 5.03. It's buffering is a little higher than many pale malts but not inordinantly so. Whereas it only takes 59.6 mEq of protons to get 6.4 kg of this malt to pH 5.5 it would take 237 to hit 5.03. With the other malts you would need a total of 260. You really need go no further to see that 5.03 is an absurd prediction. That acid would have to come from somewhere. If we ask the program to tell us how much chocolate malt would be needed to furnish it it responds that 10.2 kg which would be 54.1% of the grist would have to be chocolate. It's clearly more reasonable to ask how much lactic acid that's equivalent to. The answer is 23.6 mL of 88% strength.
What about some other malts? With Crisp's Maris Otter the lactic requirement would be 21.9 mL and with Munton's MO 27.5.
In summary: Your mash pH is going, most likely, to be in the 5.4 - 5.5 range. Do a test mash to verify this. You didn't put in any numbers wrong. The particular program you chose doesn't handle this mash very well because of the inherent flaw in its prediction algorithm (which may have been fixed) and its poor modeling of actual malt parameters. All existent programs suffer to some extent from one or both these problems.












![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)
















































