jabraben
Active Member
I've moved to the country and need to adjust my well water for brewing. I've always been fortunate to have seemingly decent city water for brewing so I've ignored water chemistry till now. I've been reading Palmer and Kaminski's book and Martin Brungard's water knowledge page but I need to brew sooner than I can effectively process all of that so I'm hoping for some help on a few points working through Bru'n Water to determine my adjustments. I've attached the Water Adjustment, Sparge Acidification, and Mash Acidification worksheets.
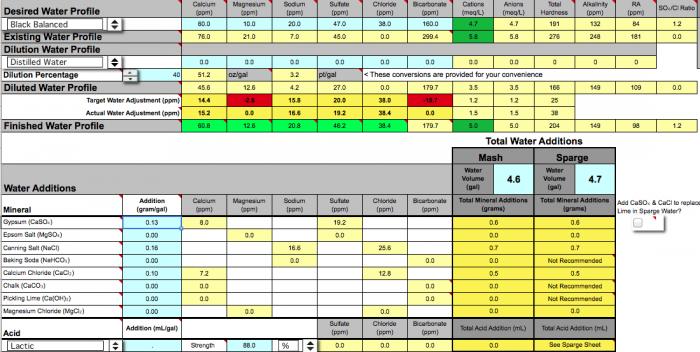
Does anything look amiss in the adjustments?
I presume that on the sparge acidification sheet I enter the alkalinity from the diluted water profile number, correct?
The starting water pH on the sparge acidification sheet is set to the pH reported on my water profile and I just want to make sure that it shouldn't be another number since I'm cutting the sparge water 40% with distilled. My impression is that distilled is neutral and consequently doesn't effect the pH of my well water but I'm not confident it has no effect.
Would that amount of lactic acid be appropriate, in terms of properly acidified sparge-water and flavor imparted in the final beer?
I should say that I'm batch sparging and I've read conflicting recommendations about the need to acidify sparge water for batch sparging, though clearly high alkalinity seems to necessitate it.
Thanks in advance for any assistance.

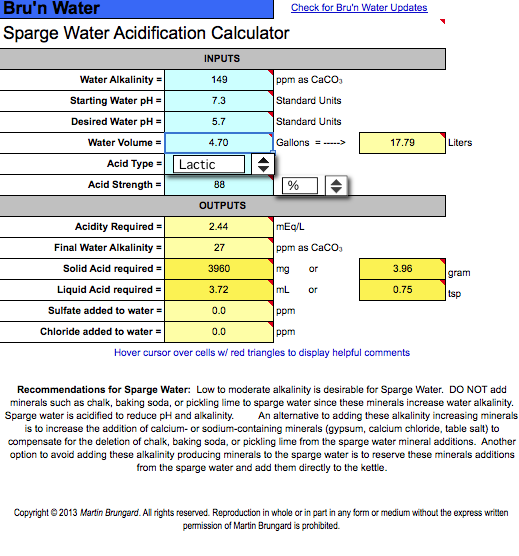

Does anything look amiss in the adjustments?
I presume that on the sparge acidification sheet I enter the alkalinity from the diluted water profile number, correct?
The starting water pH on the sparge acidification sheet is set to the pH reported on my water profile and I just want to make sure that it shouldn't be another number since I'm cutting the sparge water 40% with distilled. My impression is that distilled is neutral and consequently doesn't effect the pH of my well water but I'm not confident it has no effect.
Would that amount of lactic acid be appropriate, in terms of properly acidified sparge-water and flavor imparted in the final beer?
I should say that I'm batch sparging and I've read conflicting recommendations about the need to acidify sparge water for batch sparging, though clearly high alkalinity seems to necessitate it.
Thanks in advance for any assistance.