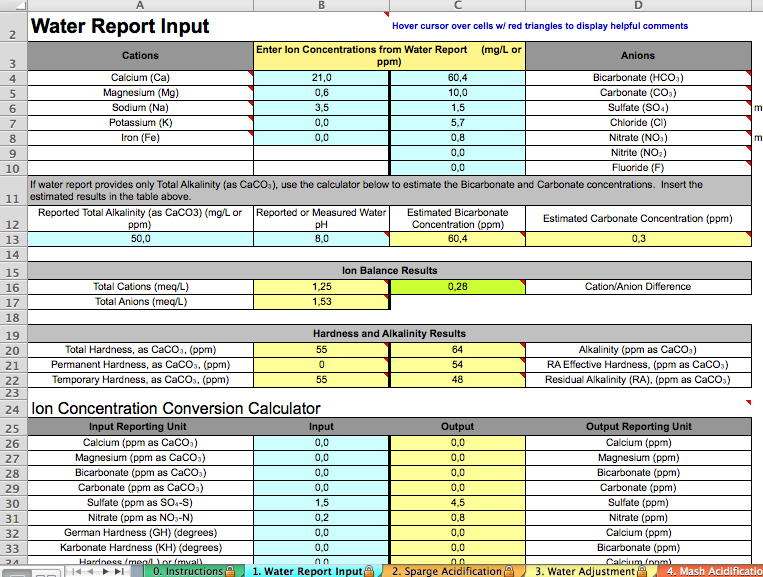
Attached is what I've typed in according to instructions from a thread about my water report from a long time ago. I was told it's basically RO water. First question, does it look right?
Second question: I'm planning on brewing a Bohemian Pilsner this weekend. I normally add acid malt when needed in order to drop the estimated pH into the range that I'm hoping for (anywhere between 5.2 and 5.5 depending on the style). I had planned on adding acidulated malt to this one too (dropping pH to 5.5), but I'm wondering if I should add any other minerals, or if I should just go with what I had originally planned since this is my first time attempting the style?
Any help is much appreciated as I know pretty much nothing about the subject of brewing water!
Second question: I'm planning on brewing a Bohemian Pilsner this weekend. I normally add acid malt when needed in order to drop the estimated pH into the range that I'm hoping for (anywhere between 5.2 and 5.5 depending on the style). I had planned on adding acidulated malt to this one too (dropping pH to 5.5), but I'm wondering if I should add any other minerals, or if I should just go with what I had originally planned since this is my first time attempting the style?
Any help is much appreciated as I know pretty much nothing about the subject of brewing water!



