I used the EZ Calc 2.0 for the first time on my last brew because my water was off quite a bit and seems to be affecting my efficiency. I was wondering if someone would mind going over my #'s and let me know if this is correct. The beer I made was awful and tasted like paint thinner. I think I may have just gotten a bug, but I want to make sure it wasn't the water additions before I make another beer.
I added the mash and sparge additions for a total of: 5g Gypsum, 18g Calcium Chloride, 13g Epsom salt. I split up the grams for 10gallons and 7gallons.
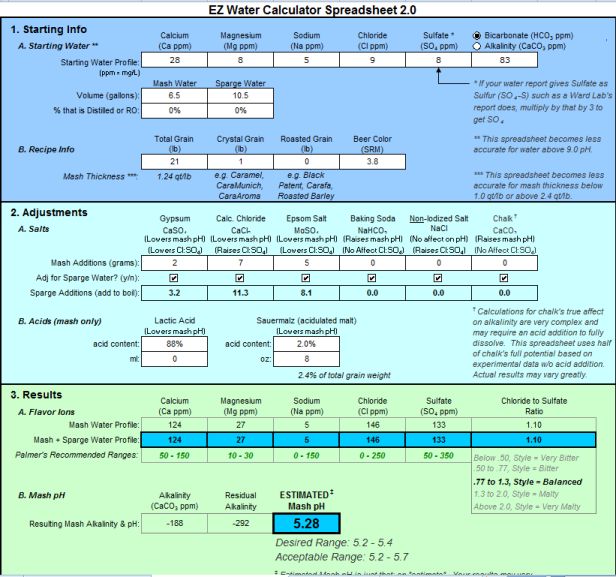
I added the mash and sparge additions for a total of: 5g Gypsum, 18g Calcium Chloride, 13g Epsom salt. I split up the grams for 10gallons and 7gallons.



