tamoore
Well-Known Member
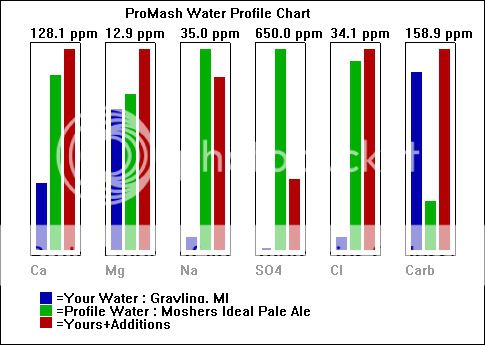
pH 8.0
Total Dissolved Solids (TDS) Est 161
Electrical Conductivity, mmho/cm 0.27
Cations / Anions, me/L 3.0 / 2.8
ppm
Sodium, Na 2
Potassium, K < 1
Calcium, Ca 42
Magnesium, Mg 9
Total Hardness, CaCO3 143
Nitrate, NO3-N 0.7 (SAFE)
Sulfate, SO4-S 3
Chloride, Cl 2
Carbonate, CO3 6
Bicarbonate, HCO3 140
Total Alkalinity, CaCO3 125
"<" - Not Detected / Below Detection Limit
Is this good water, bad water, or just OK?
Thanks for any guidance.
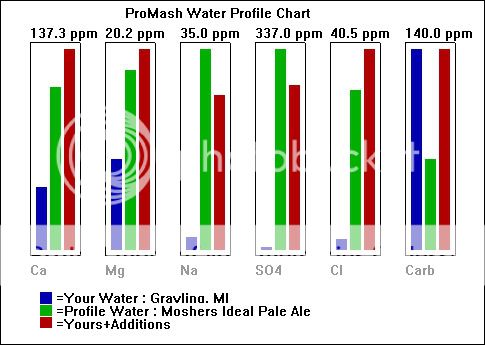
Total Dissolved Solids (TDS) Est 161
Electrical Conductivity, mmho/cm 0.27
Cations / Anions, me/L 3.0 / 2.8
ppm
Sodium, Na 2
Potassium, K < 1
Calcium, Ca 42
Magnesium, Mg 9
Total Hardness, CaCO3 143
Nitrate, NO3-N 0.7 (SAFE)
Sulfate, SO4-S 3
Chloride, Cl 2
Carbonate, CO3 6
Bicarbonate, HCO3 140
Total Alkalinity, CaCO3 125
"<" - Not Detected / Below Detection Limit
Is this good water, bad water, or just OK?
Thanks for any guidance.