If this is too risky I will not brew today. I have been brewing with this water for a few years now with only a charcol filter and the beers turn out pretty good but always leave me wanting to change something.
No - not too risky. Only a few years ago you would be told you have great water and that you should be able to brew almost anything you like with it and you have demonstrated that this is the case. Today with RO/DI it is possible to have virtually any ion profile you like and so we can make recommendations tailored to your requirements/desirements.
-Would you avoid brewing until you had a PH meter in your hand or would you feel confident basing everything off of the bru'n spreadsheet if you were in my position?
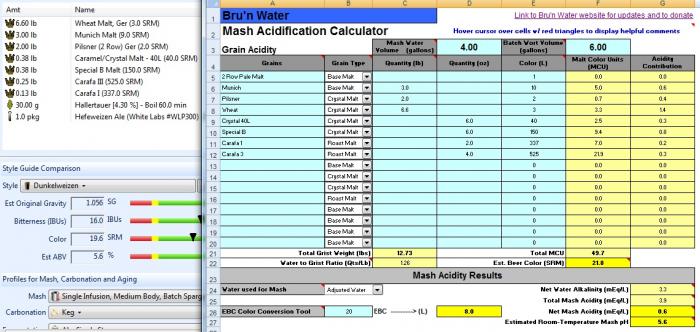
I'd go ahead. The dark beers are the riskiest because of uncertainty/variability in the malt acidities. If the spreadsheet predicts 4.8 I'd look at the amount and type of dark malts you are using. If it predicts 5.4 - 5.6 then I think you'll be OK.
-You say you traditionally mute as much of the sulfate as possible for your beers but up the Chloride and Calcium. How concerned should I be about the SO4:Cl ratio?
I wouldn't worry about it at all. Write it down in your logbook as additional data but don't let it drive your decisions. Forget about this 'malt forward'/hops forward stuff. YOu get malty beer by using more malt of the correct type. You get hoppy beer by using more hops of the correct type in the correct amount. I think the implication that you can dial in a desired hops/malt experience by setting the sulfate to chloride ratio is one of the cruelist hoaxes ever perpetrated on home brewers.
in your post on another thread you state:
Would this apply to german ales and lagers as they are generally all malt foward? also do you ever maintain a higher sulfate if you were brewing an IPA do your hop foward beers turn out fine without the sulfates?
As you know some German lagers are made with high sulfate waters and turn out fine. The brewers of those beers know what hop varieties to use and in what kind of charges with the water they have to brew with. I do Kölsch and the rare wheat beer with 0 (or as low as my RO system can get them) sulfate water the same as my Pils, Helles, Bock, Vienna. I do British ales only rarely and there I will go with the tap water which is 27 ppm. I've done pair comparisons for water classes in which I do an ale with 27 ppm and one with Burton level sulfate and everything else as ceteris paribus as I can manage. I've done this twice and the results were the same both times: the low sulfate beer was judged better but less authentic than the other. I'm associated with a brewpub where the guy cranks out really hoppy beers which the patrons love. His sulfate level is 47 and he doesn't supplement (AFAIK) being of the Michael Lewis 'your water is your terroir' school. So I'm pretty convinced that the notion that you must have high sulfate in British ales really needs to be looked at. But I am not recommending 27 or 47 or any other number as the 'proper' level for sulfate. It really is a matter of personal taste. I recommend experimentation.
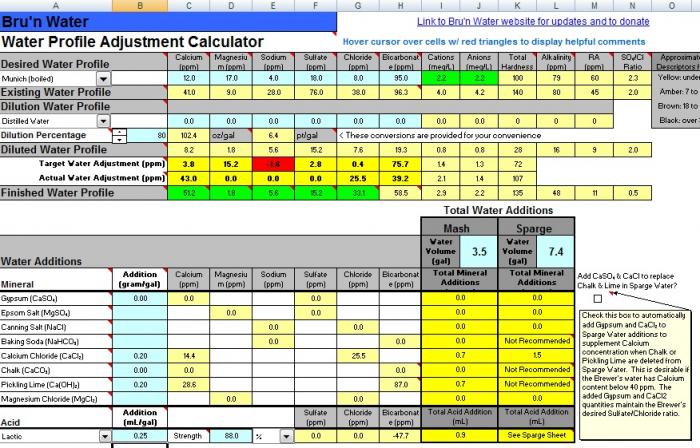
-I see that the munich profiles that I have read up on are high in bicarbonates (150+ppm) Bru'n water spread sheet states that the bicarbonates are generally undesirable.
That's generally true. The only place you want bicarbonate is if you are brewing a beer based on a style that used a lot of dark malt to combat the bicarbonate in the local water and you want the roast/toast flavors. Dunkles could be an example of that.
From what I understand the are necessary buffers to prevent an unstable mash.
Umm - not so sure about that. Biarbonate/carbonic buffers to pH 6.38 and mash pH is often within 1 pH of that and the rule of thumb is that a buffer should be designed for a pH less than one unit away from the pK (6.38) so in that sense I suppose you could consider the buffering capacity of the carbonic/bicarbonate system but I generally think of bicarbonate as a base that is stressing the buffers of the malt by trying to pull pH higher than I want. I often say the first of the cardinal rules of brewing is 'alkalinity = bad'.
If I am brewing a light beer (hefe) should I back off on the pickling lime additions to maintain a lower bicarbonate,
For lighter beers you should avoid alkali (pickling lime, bicarbonate, carbonate) alltogether. You will need acid for these beers, not alkali,
and for darker beers (dunkelweizen/munich dunkel) up the bicarbonates with lime to have a higher buffering power for the darker malts?
Possibly but I always say that one should never (this is the second cardinal rule) add alkali unless it is confirmed by pH meter reading that it is necessary.
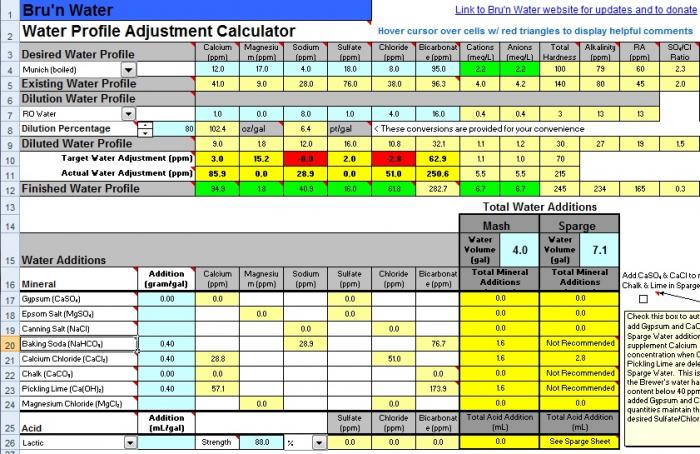
this new attachment is profiled with my dunkelweizen malts and lands me at 5.4 mash ph. would you remove the pickling lime from the equation and bump the cacl? When I increase CaCl to .5g/gal it takes my calcium to 44.2ppm and the chloride to 71.4ppm.
Yes because I believe the risks of going too high in pH by adding alkali are higher than the risks of going too low by omitting it but there is a risk that you will go too low. Things depend on how much of which dark malts you are using.
I do not have a good understanding of the relative terms "high" or "low" and cannot tell if the Ca or Cl would be too high or that the bicarbonates would be too low without the lime.
The calcium and chloride levels look just dandy.
also am I correct that wheat malt not add to the malt acidification?
No - not compared to what sauermalz does if that's what you are asking.