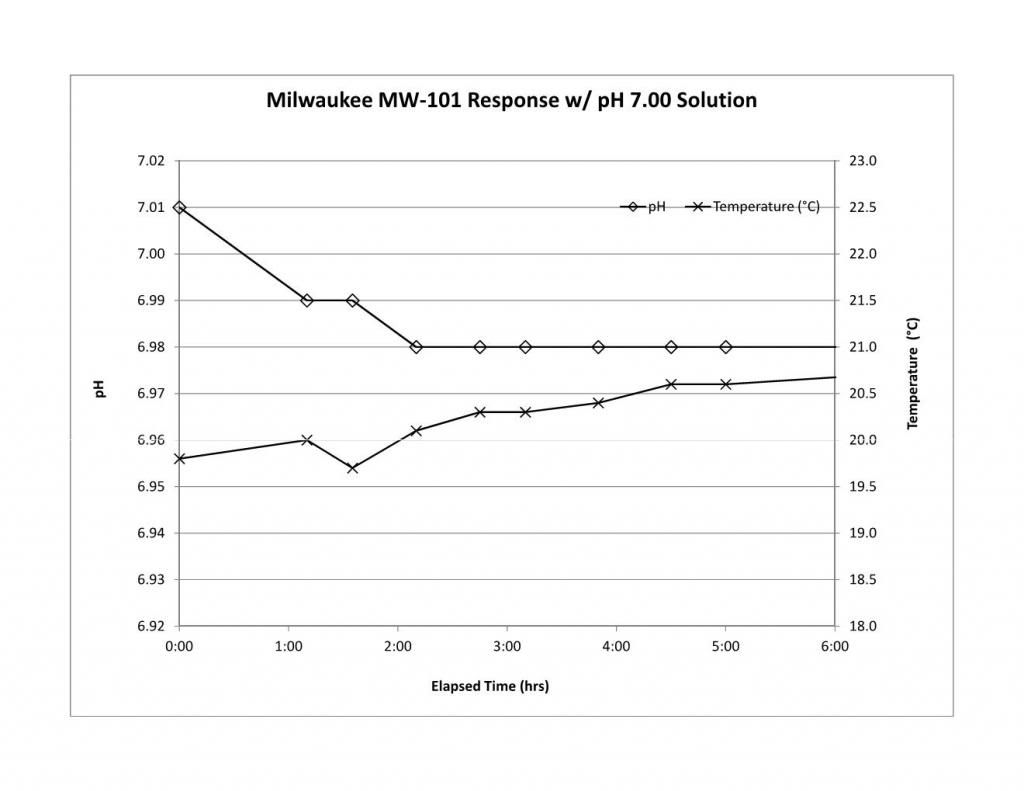
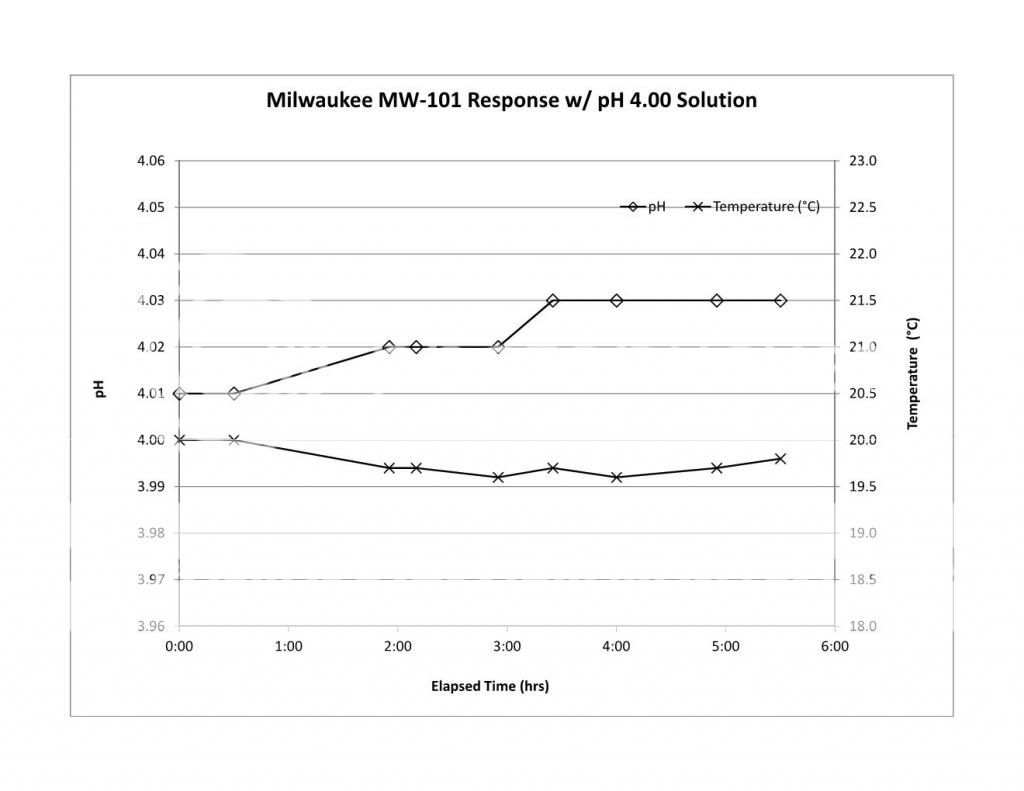
So what meters are recommend? (I am leaning toward the milwaukee - Model MW101)
From the discussion
Hach PocketPro+ $110 (best place to buy? Looks like direct)
Replacement pH probe is $67
In the first post here I describe calibration and stability checks which, if passed, indicate that the meter is suitable for brewing provided, of course, that it has good reliability.
This new Hach unit and a Hanna pHEp meter are the only ones on which I have done cal and stability checks. Based on my findings on the Hach, at least two other people on here have bought them and done the stability checks. This unit looks good - so far. But we don't know how robust the thing is, how long the electrode is expected to last... I showed it to the brewer at Gordon Biersch yesterday and he went off and ordered one on the spot. He'll certainly beat it up in his normal use of it and has promised to let me know how it does for him. If he is happy with it at the end of year then my recommendation for it will go from tentative to definite.
I have also done stability checks on the Hanna pHEp. It is quite stable but you can't calibrate it because it decides for itself when to accept the calibration reading and it does so too soon. This can be worked around quite simply by subtracting the average cal check (4 and 7 pH) error to all subsequent readings but I don't recommend this meter simply because people who are using a pH meter for the first time get confused enough about having to do cals as it is.
Thoughts on the below
Milwaukee MW101 pH Meter w/Battery $80 (2 point) - BNC pH Probe Style
RESOLUTION 0.01 pH
ACCURACY (@25°C) ±0.02 pH
http://www.bulkreefsupply.com/catalog/product/view/id/709/
I have not done stability or calibration checks on the MW101 nor have I seen anyone else publish data. I have never had one in my hands. What is disturbing about this meter is that it gets an average review score of 2.5. Half of the reviews are 5 stars and the other half 0 stars. It seems that you either get a meter you like or a complete piece of junk with the chances being about 50/50. Another 'problem' is that these meters are analog. The advantages to digital are well known (though some stubbornly refuse to accept them) and as I have set them out so many times before, I won't do it again. I put problem in quotes because you can very well use an analog meter with manual TC (provided you are in the 50% that get a working meter). People did it for years. There is an MW102 which is the digital version. I have no stability or cal check data on it either and don't know anything about its reliability.
(You can get 2 Wire pH Lab Probes from HongKong for < $20 that work great 3 months and fine so far with this on my Marine Tank)
- http://www.ebay.com/itm/251124829815?ssPageName=STRK:MEWAX:IT&_trksid=p3984.m1438.l2649
This is just an electrode - you still need a meter (and a temperature probe unless you want to do TC manually). The electrode is, of course, where the rubber meets the road. This one doesn't have a particularly good spec.
& Here at the top 2 that get good reviews on the Marine Side ...
I can't comment on any of these. I look for laboratory grade instruments as in mash pH measurement you really want to be as accurate as possible. When I see a laboratory grade instrument maker's label (Hach) on a $125 meter I get excited and hope wells, as they say, eternal. But I'm also a cynical SOB. If the Hach turns out to have shortcomings comparable to those I know about in other inexpensive meters I'll throw it on the heap with them.