- Joined
- Apr 25, 2009
- Messages
- 962
- Reaction score
- 2
Anyone know what profile I should go by?

From Pale Ales by Terry foster
Ca 50 - 100 ppm, SO4 100 - 200 ppm, Cl 20 ppm
I've been using his recommendations for some time now, and find them excellent.
-a.
I actually go with 155 Cl and 182 SO4 and I find that my APAs do not have the necessary fruitiness.
Do you think I should lower the Cl to a minimum?
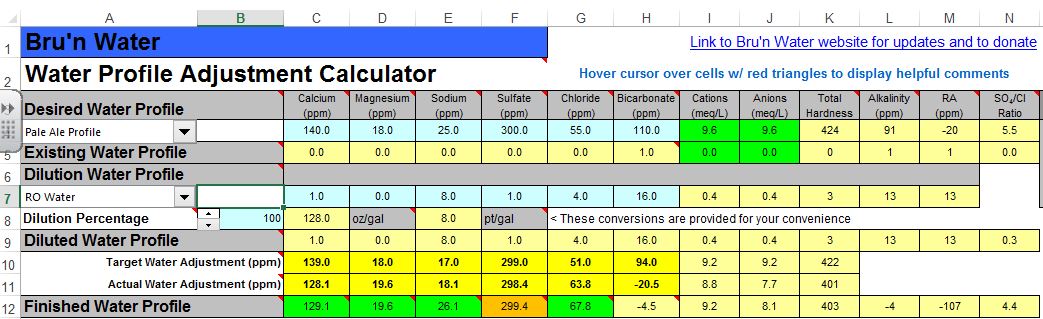
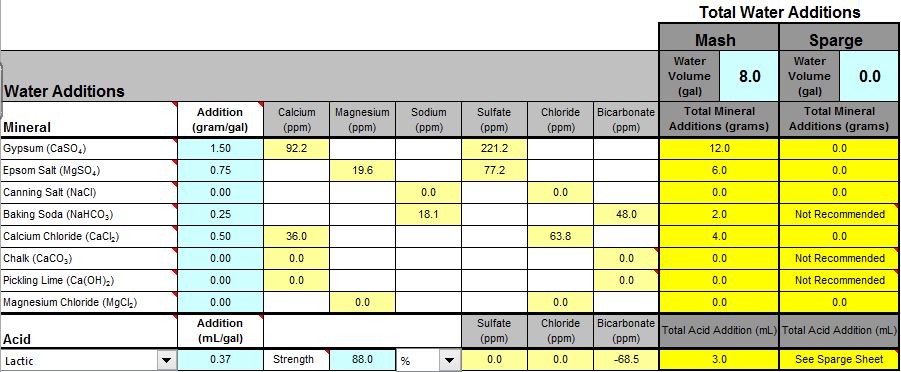
From the screenshot, it looks like you're adding both baking soda, and lactic acid? You don't want to do that. Remove the baking soda and see where the mash pH is, and go from there.
This thread is nearly 2 years old, so I feel secure in jacking it.
2 questions about nailing a pale ale profile:
1) I only have Calcium Chloride and Gypsum at my disposal, can I match this profile without raising my Chloride levels too high? Oops. Edit: I forgot about epsom salts. I can get that easily and that seems to boost my sulfate quite a bit
2) I like a pale ale that balances the hops and a malty backbone. With Brunwater's Pale Ale profile, the SO4/CL ratio is 5.5 (VERY bitter territory). Soothe my fears, if possible. How much does the ratio matter? I read AJ's sticky on the subject, but it doesn't really answer my question.
Edit: New screen shots


@Yooper, @mabrungard ?
Thanks guys.
Well it often isn't retained that long and it's the magnesium. Think milk of magnesia (Mg(OH)2), magnesium citrate and what they are used for.Caused water retention in the gut which is what epsom salt does,
Not too surprising that lots of minerals lend a mineral taste...and the beer had an artificial mineral taste to it.
I think this is very wise and often advise people to start out with very low levels of both sulfate and chloride or even just chloride and build up after tasting dosed finished beer.I also learned is to establish a known ratio for a beer style, start on the lower end of the scale and add the missing qualities of minerals to your beer upon tasting.
I was thinking of using the baking soda for the sodium content.
I know that the baking soda and acid is redundant as far a PH goes.
Assuming you want a mash pH of 5.5 and 1 mol (23 mg) of sodium per L you could add 1 mmol of NaHCO3 and 0.91 mmol of lactic acid to each liter. You would have 0.12 mmol/L bicarbonate and 0.91 mmol of lactate ion in the water.
Hi, I am also trying to get my water to a good APA profile. I have been disappointed with the various all grain Pale Ale attempts I've made, I'm concerned the water maybe a contributor.
You'd measure the bicarbonate or sodium lactate with a balance and the lactic acid (which brewers usually buy as a solution) with a pipette though it can be measured with a balance as well.
I have lactic acid and a dropper. But a balance like this ?
https://www.google.ca/search?q=bala...UIBygB&biw=1366&bih=632#imgrc=EVobfbK28ixLvM:
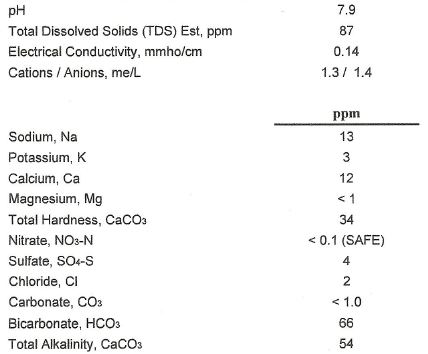
I'm hoping that you added some degree of mineralization to that water. While that water is a great starting point, it could use more mineralization for many beer styles. I find this is especially true when brewing APA or IPA. They would be a bit bland if brewed with that unadulterated tap water.
Hey just out of curosity, where do you obtain the figures for your water? Send it off to a Lab? Or maybe you have an in house kit?
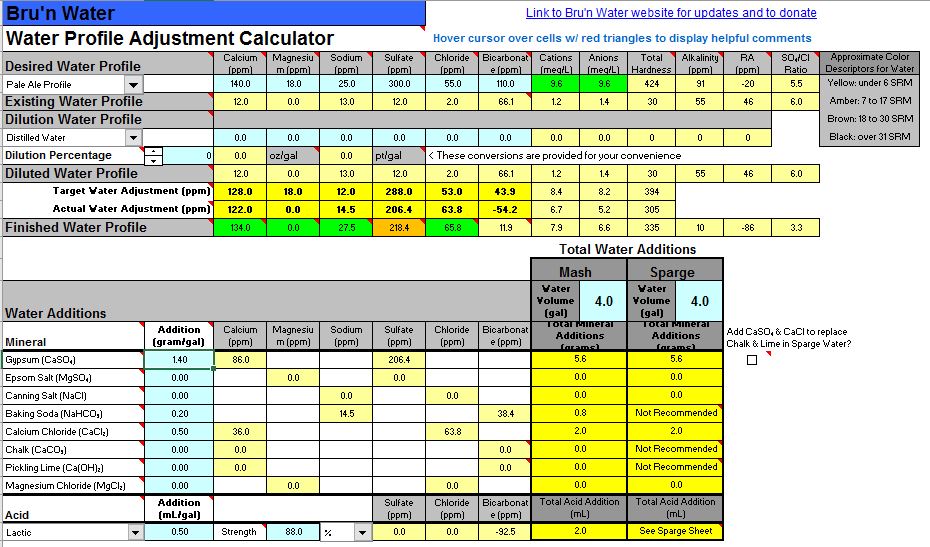
Capisco but it sort of says 'This spreadsheet has an error. What else is wrong?' I'd rather put blank fields than something that is obviously wrong. Perhaps put a solid field (no number) of a particular color with an annotation, and/or general comment in the instructions, that says when you wave your credit card you get to see what's behind the green doors.Yes, the free-version of the sheet doesn't include the proper accounting of the added acid anion. It is an otherwise innocuous error that doesn't affect the results.
I use Randy Mosher's profile for Pale Ales for APAs and IPAs.
Ca: 110
Mg: 18
Na: 17
Cl: 50
SO4:350
CaCO3: 57
Are those figures in grams for a 5 gallon batch or are they in ppm?
Also, if it's in PPM, that is equal to mg/ litre. So I should just multiply by the number of litres of the total water used (mash+sparge) to arrive at amounts of salt needed ,right?
Those numbers @studmonk3y quoted are in ppm.
Sort of. You can't add any of these cations or anions without also adding an associated anion/cation. Examples: to add calcium, you have to add it as calcium chloride, or calcium sulfate, or even calcium hydroxide.
So to do the math, you have to know what percentage of the weight of the salt is made up of the ion you are targeting. And when building a whole profile, the math is cumbersome. That's why there are several software options, some free, that make it easier.
Enter your email address to join: