BrewThruYou
Well-Known Member
Looking for some feedback...I've been reading tons on water treatment and I hopefully have my additions down. Can someone look this over for a FW Union Jack IPA?
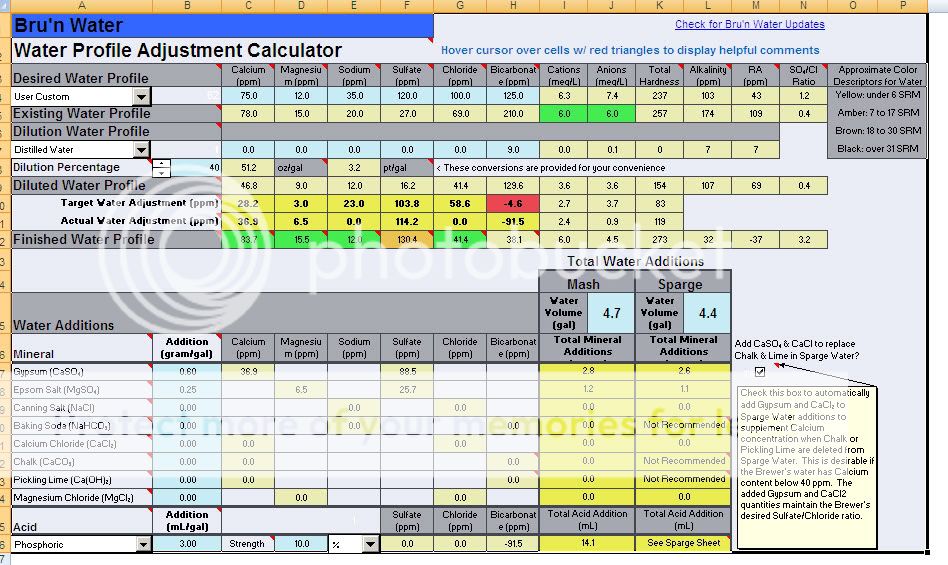
Water is very alkaline:
Ca: 78
Mg: 15
Na: 20
K: 2
HCO3: 210
SO4: 27
Cl: 69
Alkalinity: 172
Total Hardness: 258
I'm shooting for a Firestone Walker mineral profile - around 120ppm sulfate and 100ppm chloride. Going to cut with 40% distilled and add 0.6g Gypsum and 0.25g Epsom Salt per gallon. Grain bill is 11.75# of 2-row, 2lb Munich, 0.75# Carapils, 0.25 Crystal 45. 14.1ml of 10% phosphoric acid in the mash shows 5.4 PH. I do have a PH meter and will take a reading. Then 22ml of 10% phosphoric in the sparge water.
I have two questions:
1. Is this too much phosphoric acid? I'm assuming it's not since it has a cleaner taste than the other acid options (I might try getting 85% phosphoric next time from dudadiesel).
2. If I'm batch sparging, do I still need to bring sparge water under 6? I assume that it's more critical for fly spargers.
Here's my Brun Water screenshot.
Water is very alkaline:
Ca: 78
Mg: 15
Na: 20
K: 2
HCO3: 210
SO4: 27
Cl: 69
Alkalinity: 172
Total Hardness: 258
I'm shooting for a Firestone Walker mineral profile - around 120ppm sulfate and 100ppm chloride. Going to cut with 40% distilled and add 0.6g Gypsum and 0.25g Epsom Salt per gallon. Grain bill is 11.75# of 2-row, 2lb Munich, 0.75# Carapils, 0.25 Crystal 45. 14.1ml of 10% phosphoric acid in the mash shows 5.4 PH. I do have a PH meter and will take a reading. Then 22ml of 10% phosphoric in the sparge water.
I have two questions:
1. Is this too much phosphoric acid? I'm assuming it's not since it has a cleaner taste than the other acid options (I might try getting 85% phosphoric next time from dudadiesel).
2. If I'm batch sparging, do I still need to bring sparge water under 6? I assume that it's more critical for fly spargers.
Here's my Brun Water screenshot.



